Introduction to neutrophils
Neutrophilic polymorphonuclear leukocytes or poly-morphonuclear neutrophils (PMNs) play a crucial role in host defense by phagocytosing and killing the invading microorganisms, and in the pathogenesis of inflammatory diseases 1, 2. Neutrophils accumulate at the site of inflammation and can promote vascular injury through the secretion of granule constituents, reactive oxygen metabolites, and phospholipase products, resulting in local edema, thrombosis or hemorrhage. Neutrophils have intracellular vesicles or granules that secrete proteins by a process referred to as exocytosis or degranulation, which is of particular significance with respect to tissue damage in inflammatory diseases. The cytoplasmic granules of neutrophils are involved in a wide spectrum of functions including, engulfment and killing of foreign particles and pathogens, cell-cell interaction and adhesion, cell signaling, modulation of the surrounding environment, and transendothelial migration. As high amounts of deleterious constituents are stored inside the neutrophilic cytoplasmic granules, hence their release must be perfectly controlled in order to avoid tissue injury. Exocytosis involves the fusion of granules with the plasma membrane, leading to the release of granule contents and exposure of granule membrane proteins at the cell surface.
Neutrophil structure
Neutrophils are the most abundant type of granulocytes. They constitute about 70% of the white blood cells. Their diameter ranges from 12-15 µm. Neutrophils are named so because of their neutral staining with Wright stain. They have nucleus divided into 2-5 lobes. The multilobed nucleus contributes to the extreme elasticity of the cell, which is important for the cell to make the rapid transit from the blood vessels through tight gaps between the endothelial cells during transendothelial migration. In the cytoplasm of PMN’s the Golgi apparatus is small, mitochondria and ribosomes are sparse and rough endoplasmic reticulum is absent. Cytoplasm contains abundant secretory vesicles.
Secretory vesicles:
They are the most important components of the neutrophil structure. Secretory vesicles have an endocytotic origin 3 and they represent a pool of membrane-associated receptors, that get incorporated into the plasma membrane after the release of the vesicles 4. The most abundant receptors within the secretory vesicle membrane are ……..Contents available in the book……..Contents available in the book……..Contents available in the book……..Contents available in the book…….
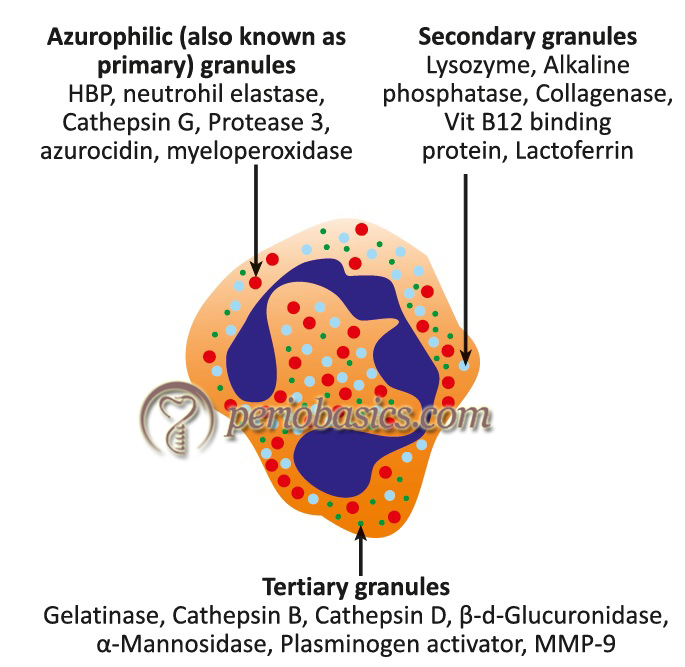
Neutrophils contain releasable membrane-bound secretory vesicles or phosphasomes. There are three major functional types of granules in the neutrophils, namely: azurophilic or primary granules, specific or secondary granules, and gelatinase-containing tertiary granules 8-12. Some proteins have been localized in additional cytoplasmic organelles that do not perfectly fit with the above mentioned major cytoplasmic granule populations, including organelles containing transforming growth factor-α 13, and vesicles containing tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) 14. A brief description of the primary, secondary and the tertiary granules of neutrophils is as follows,
A. Primary or azurophil granules: Azurophilic granules are characterized by their content of myeloperoxidase and β-glucuronidase. Azurophil granule degranulation is confined primarily to internalized phagocytic vacuoles during phagocytosis 15, indicating that this granule is mainly involved in phagocytosis.
B. Secondary or specific granules: Markers from specific granules have lactoferrin and vit-B12 binding proteins. Because specific granules contain four types of extracellular matrix receptors (laminin, fibronectin, vitronectin receptors, as well as the receptor for C3bi/fibrinogen CD11b/CD18), they have been named as “adhesomes” 16.
C. Tertiary or secretory granules: Tertiary granules contain adhesion proteins (e.g. CD11b/CD18) 17, 18 as well as heparanase and gelatinase, two major extracellular matrix degradative enzymes involved in extravasation process 19. Tertiary granules are most rapidly released.
Neutrophil homeostasis
Neutrophils are the primary leukocytes recruited in the gingival crevice in response to the bacterial biofilm 20, 21. They make the primary line of defense against microorganisms present in the dental plaque. Their absence or excess, both result in damage to the host tissue, thus the number of neutrophil and their distribution are very important for the maintenance of periodontal health. Let us try to understand the biology of the production of neutrophils, their trafficking, and their clearance.
Neutrophils are produced in the bone marrow from where they are released into the circulation. The granulocyte colony-stimulating factor (G-CSF) plays a primary role in the granulopoiesis and neutrophil release 22. A large number of mature neutrophils are retained in the bone marrow, which is facilitated by interactions between CXC chemokine receptor 4 (CXCR4) on neutrophils and chemokine CXCL12 (stromal-derived factor-1/SDF-1) produced by the bone marrow stromal cells. G-CSF interferes with the CXCR4-CXCL12 interactions, thus facilitating the release of neutrophils from the bone marrow 23. Interleukin-17 (IL-17) up-regulates G-CSF, which in turn promotes granulopoiesis and neutrophil release from the bone marrow 22. At an inflammatory site, neutrophils attract IL-17-producing CD4+ T-lymphocytes, which facilitate recruitment of neutrophils 24. Furthermore, neutrophils release CCL2 and CCL20 chemokines, which are ligands for the CCR2 and CCR6 chemokine receptors on Th17 cells. Thus, neutrophils promote Th17 cell accumulation at the site of inflammation. In turn, Th17 cells recruit more neutrophils by producing IL-17. Thus, a positive loop is formed between neutrophils and Th17 cells 25, 26.

After neutrophils enter the circulation, they quickly mobilize to the site of inflammation where they transmigrate ……..Contents available in the book……..Contents available in the book……..Contents available in the book……..Contents available in the book…….
The clearance of neutrophils takes place by their phagocytosis by the phagocytes, such as macrophages and dendritic cells in the tissue. This process not only removes the old cells, but also regulates the production of new neutrophils. Phagocytosis of an apoptotic neutrophil triggers an anti-inflammatory response which is mediated by the reduced production of IL-23 by macrophages. As IL-23 induces the production of IL-17 by many cells of the immune system, its reduced production down-regulates IL-17 production. The reduced IL-17 levels lead to less G-CSF production and as a consequence, there is less neutrophil production 28. This mechanism of feedback control over neutrophil production is referred to as “neutrostat” (neutrophil rheostat) which maintains steady-state neutrophil levels in the circulation. The senescent neutrophils return to the bone marrow for clearance after they have an increased expression of CXCR4. The CXCR4 expression increases on neutrophils as they age.
Neutrophil function during periodontal inflammation
As discussed earlier in “Host-microbial interactions in periodontal diseases”, PMN’s are one of the first-responders of inflammatory cells to migrate toward the site of periodontal inflammation. This migration of cells is mediated by chemoattractants such as IL-8 secreted by oral epithelial cells, connective fibroblast and immune cells 29. There are multiple mechanisms by which neutrophils exert their antibacterial activity, including phagocytosis, the release of antimicrobial substances, and the formation of neutrophil extracellular traps (NETs) 30. Neutrophils produce various proteinases which cause damage to the host tissues and produce various chemical mediators which influence the inflammatory as well as the immune response 31. Following is a brief description of neutrophil functions,
Adherence:
The surface of neutrophils is coated with surface adhesion molecules. These adhesion molecules interact with intracellular adhesion molecule (ICAM)-1 and 2 on endothelial cells. ICAM-1 and ICAM-2 belong to the immunoglobulin superfamily. The expression of ICAM-1 is induced by tumor necrosis factor (TNF), interlukin-1 (IL-1) and interferon- γ (IFN-γ). Adhesins on the surface of PMN’s are composed of a group of glycoproteins referred to as α and β subunits. β subunit is known as CD18 and α subunit is known as CD11. The α subunit is found in 4 forms viz. a,b,c and d. So, based on α subunit variability, glycoproteins are classified as CD11a/CD18, CD11b/CD18, CD11c/CD18, CD11d/CD18 (see “Basic concepts in immunity and inflammation” for more detail).
Chemotaxis:
The invading microorganisms are coated with plasma proteins like, IgG or C3B. This process is called opsonization. Opsonization done by IgG and IgM is heat stable, whereas opsonization done by C3b and ic3b i.e. compliment is heat-labile.
Receptor for IgG on neutrophil: Fcγ
Receptor for C3b on neutrophil: Cr1
The mechanism of neutrophil chemotaxis has been described in detail in “Host-microbial interactions in periodontal diseases”.
Microbial killing by phagocytosis:
It involves the fusion of phagosome with the neutrophilic granules, leading to the discharge of granule contents into phagosome, resulting in the intracellular killing of bacteria. The important step in this process is the recognition of the particle as foreign by the neutrophil. The interactions between opsonins IgG and C3b and their receptors on the surface of the neutrophil are involved in this step 32. These interactions result in the ……..Contents available in the book……..Contents available in the book……..Contents available in the book……..Contents available in the book…….
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Respiratory burst:
The formation of H2O2 and superoxide anion constitutes the phenomenon of the respiratory burst. Neutrophil stimulation results in a sharp increase in oxygen consumption by the cell. Cell activation leads to the release of an enzyme, NADPH oxidase from the cytosolic side of the plasma membrane. NADPH is oxidized to NADP on the outer surface of PMN. This leads to the production of superoxide anion (O2-) on the cell surface. Superoxide is converted into H2O2 by the action of enzyme superoxide dismutase. H2O2 in itself is not damaging to the tissues, but when it combines with myeloperoxidase (azurophilic granule enzyme) and halide ions from the plasma membrane, the result is the formation of hypochlorous acid, which is a potent oxidant and very toxic 40, 41. The myeloperoxidase system is effective in killing bacteria, fungi, viruses, and mycoplasma. It is also capable of destroying the tissues. The reactions of the respiratory burst can be summarized as follows,
First step involves the reduction of O2 molecules:
2O2 + NADPH → 2O2− + NADP− + H+
Next, O2− dismutates to hydrogen peroxide (H2O2) and the azurophil granule enzyme myeloperoxidase catalyzes the oxidation of Cl− by H2O2 to yield hypochlorous acid (HOCl):
2O2− + 2H+ → H2O2 + O2
H+ + Cl− + H2O2 → HOCl + H2O
Neutrophil extracellular traps (NETs):
This mechanism of microbial killing by neutrophils has been recently explained 27, 42, 43. In this mechanism, the nuclear and mitochondrial DNA is released into the extracellular space. It is an active process and involves the activation of NADPH oxidase, histone hypercitrullination, and decondensation of chromatin. These DNA strands and filaments containing high local concentrations of formerly intracellular antimicrobial proteins collectively form the neutrophil extracellular traps (NETs) 44. This process is also referred to as NETosis. This mechanism facilitates immobilization of a vast number of microorganisms by neutrophils, which otherwise may overwhelm their phagocytosis capacity. Furthermore, NETs prevent further spread of entrapped microorganisms into the surrounding environment and blood stream. Various initiators of NETosis include bacterial cell wall components that activate complement receptors, Fc receptors, or Toll-like receptors on neutrophil surfaces.
Action of neutrophils on biofilm
Earlier, it was believed that neutrophils cannot affect the microorganisms in the biofilm. However, recent investigations have shown that neutrophils do exert their antimicrobial activity on microorganisms in a protected environment of biofilm. As already discussed in the previous chapter, neutrophils are attracted towards the site of infection by various host and bacterial derived chemoattractants. The epithelial cells, other immune cells or neutrophils themselves secrete molecules that act as chemoattractants, facilitating neutrophil migration 45, 46. Small QS (quorum sensing) molecules of the N-acyl homoserine lactone (AHL) family as well as bacteria-derived formyl-Met-Leu-Phe act as potent chemoattractants for neutrophils 47, 48. Neutrophils identify biofilm by their receptors for lipopolysaccharides, peptidoglycans, microbial DNA, and other pathogen-associated molecular patterns (PAMPs) 49, 50. Various in vitro and in vivo studies have demonstrated the accumulation of neutrophils around or within the biofilm, demonstrating neutrophil activity against microorganisms in the biofilm 51-58. Neutrophils exert their antimicrobial activity on or within biofilm by various mechanisms, including phagocytosis, degranulation, NETosis and respiratory burst.
Protective mechanism of biofilm against PMN’s
The biofilm has its own defense mechanisms against neutrophils. These mechanisms can either directly counter the neutrophils or may camouflage the biofilm. Quorum sensing plays an important role in these protective mechanisms. It has been demonstrated that quorum sensing molecules promote the production of bacterial surfactants (rhamnolipids) by P. aeruginosa biofilms and result in a rapid rate of neutrophil death 59. Furthermore, toxins produced by the planktonic cultures of S. aureus and Aggregatibacter actinomycetemcomitans have been shown to induce lysis and degranulation of neutrophils 60-62. Along with this, bacteria in a biofilm can render themselves resistant to neutrophil-mediated killing by making them non-recognizable by neutrophils. It has been shown that ……..Contents available in the book……..Contents available in the book……..Contents available in the book……..Contents available in the book…….
Neutrophil disorders
Neutropenia and Agranulocytosis:
Neutropenia may be defined as a neutrophil granulocyte count of less than 1,500/μL 75. There are three general guidelines used to classify the severity of neutropenia based on the absolute neutrophil count, measured in cells per microliter of the blood,
- Mild neutropenia, where the absolute neutrophil count is ≥ 1000 and < 1500/μL- Patient has minimal risk of infection.
- Moderate neutropenia, where the absolute neutrophil count is ≥ 500 and < 1000/μL- Patient has moderate risk of infection.
- Severe neutropenia, where the absolute neutrophil count is < 500/μL- Patient has a severe risk of infection. Neutrophil count of less than 500/μL is also referred to as agranulocytosis.
Three lines of evidence support that neutrophils protect the periodontium against infections. Firstly, primary neutrophil or myeloid abnormalities have been associated with severe periodontal destruction; secondly, otherwise healthy individuals with severe periodontal problems have a defective neutrophil function, and thirdly, experimental neutropenia in animals results in a rapid periodontal infection. Neutropenia may be primary or secondary in its etiology.
Primary:
- Genetic defect in elastase gene.
- Morbus kostmann’s syndrome (MKS).
Secondary:
- Myelosuppression.
- Drugs (idiosyncratic reactions).
- Infections.
Autoimmune disorders.
The neutropenic phase in any of the above conditions may clinically manifest as recurrent fever, malaise, headaches, anorexia, pharyngitis, bacterial infections, ulcers of the oral membrane, and periodontal disease. Because of the absence of normal neutrophil function, a rapid periodontal destruction may occur, especially in the absence of adequate oral hygiene. Following is the description of some diseases/conditions associated with neutrophilic defects which usually present with periodontal manifestations,
Chediak-Higashi syndrome (CHS):
It has an autosomal recessive mode of inheritance, localized to chromosome 1q43. In this disease, azurophilic granules and specific granules fuse to form giant granules called megabodies. Neutropenia, depressed inflammation and relative lack of neutral serine proteases occur in CHS. The formation of reduced oxygen metabolites is greatly exaggerated. Oral manifestation of this disease includes severe periodontitis and oral mucosal ulcerations. Patients usually experience gingival hemorrhage and early dental loss 76, 77. The increased number of putative periodontal pathogens, including Porphyromonas gingivalis, Prevotella intermedia, and Tannerella forsythia have been found in the periodontal site of CHS patients 78.
Specific granule deficiency:
It is a rare disease which was originally described as a disease in which neutrophils lacked specific granules. But now, it is clear that the defect is in the packaging of azurophilic and specific granule proteins. Specific granule proteins which are missing include lactoferrin, cobalophilin, cytochrome b, FPR, C5a receptor, and CR3. So, because of lack of these components, neutrophils show 79-84,
- Depressed respiratory burst activity.
- Diminished ability to respond to chemoattractants.
- Poor phagocytosis.
- Atypical nuclear morphology
The packaging of defensins into azurophilic granules is also affected. So, intra-lysosomal killing is slow. The inflammatory response is also slow due to above defects. This disease may probably have autosomal recessive expression.
Morbus kostmann’s syndrome (MKS):
Morbus kostmann syndrome (MKS) is an autosomal recessive disorder characterized by severe neutropenia that results in severe bacterial infections early in life. The primary line of treatment for MKS is the administration of granulocyte colony-stimulating factor (G-CSF). Although, in MKS patients neutrophil count comes to normal after G-CSF treatment, but they still exhibit severe periodontitis. A possibility is that neutrophils and keratinocytes lack appropriate antibiotic peptides. Thus, these patients have diminished levels of α-defensins and LL-37. α- defensins are not as effective against periodontal pathogen as LL-37.
Palmoplantar hyperkeratosis:
Palmoplantar keratodermas are a group of disorders characterized by thickening of the skin on the palms of the hands and soles of the feet of affected individuals 85. We have three related diseases in this category,
- Papillon-Lefevre syndrome (PLS).
- Haim-Munk syndrome (HMS).
- Non-syndromic prepubertal periodontitis (NS-PPP).
Both PLS and HMS show periodontitis with rapid rate of progression and palmoplantar hyperkeratosis. HMS has additional features like hyperkeratosis, arachnodactyly, acro-osteolysis, atrophic changes in the nails and deformity of the fingers. NS-PPP shows one less feature i.e. there is no palmoplantar hyperkeratosis. HMS has been traced back to Cochin, India. In PLS, HMS and NS-PPP there is allelic variation in cathepsin c gene, which is localized to chromo-some 11q14-q21. Cathepsin c is distributed to many tissues, including leukocytes. It is important in protein degradation and proenzyme activation. It has been shown to be involved in the activation of T-cell granzymes A and B; therefore it may also be significant in the activation of neutrophil serprocidins (Granzymes-A related molecules) and neutrophil cathepsin-G (Granzyme-B related molecule).
The exact defect is still unclear, but it seems that in PLS defect lies in myeloperoxidase deficiency, low integrin expression, increased superoxide production and defective phagocytosis and chemotaxis 86. Neutrophils from individuals of PLS show decreased receptor affinity for chemotaxis such as formyl-peptides 87. Other features are,
- Increased circulating NK cells.
- Decreased monocyte phagocytosis.
- Diminished lymphocyte responsiveness.
- Periodontal connective tissue clearly shows dominated plasma cells.
Other syndromes with qualitative neutrophil defects that could predispose to periodontal destruction include Kindler syndrome and Hypotrichosis-osteolysis-periodontitis-palmoplantar keratoderma (HOPP) syndrome 88.
Chronic granulomatous diseases:
It is a group of diseases characterized by the inability of the phagocytes to reduce oxygen. In this condition, there is a deficiency of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which results from a mutation in one of the four components of the NADPH oxidase complex. The most frequent form is the X-linked (XCGD), with mutations in the CYBB gene encoding gp91phox subunit. Rare subgroups are caused by mutations in CYBA, NCF1 or NCF2 genes encoding p22phox, p47phox or p67phox subunits respectively 89, 90. It is characterized by the presence of recurrent, indolent, pyogenic infections caused by catalase-positive bacteria. In addition, CGD patients also suffer from infections and sterile hyper inflammation in the oral cavity 91. Because the host phagocytes are unable to mount a normal respiratory burst, they have difficulty in ……..Contents available in the book……..Contents available in the book……..Contents available in the book……..Contents available in the book…….
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Hyper-IgE syndrome (Job’s Syndrome):
The hyper-IgE syndrome was first described in 1966 by Davis, Wedgwood and Schaller 102. It is a rare, complex disorder that has been localized to chromosome 7q21 and is characterized by a marked elevation of IgE. The exact etiology of the hyper-IgE syndrome is unclear, but heterogeneous disorders of the immune system have variably been described. These include impaired production of IFN-γ by T-cells, defective T-helper 1 (Th1)-dependent cytokine response, a skewed Th1/Th2 cell ratio, a diminished memory T-cell populations, decreased delayed-type hypersensitivity responses, an impaired response of lymph cells to antigenic and alloantigenic stimulation 103, as well as a defective neutrophil chemotaxis 104.
The patient has chronic dermatitis, coarse faces and serious lifelong recurrent infections which result in skin abscesses remarkable in their lack of erythema (“cold” abscesses). The eczematoid dermatitis starts in the newborn period and is typically associated with and driven by Staphylococcus aureus infection. Recurrent bacterial sinusitis and otitis are common in this condition. Repeated lung infections due to S. aureus, Streptococcus pneumoniae, and Haemophilus influenza are also common and start in early childhood.
Leukocyte adhesion deficiency type-I (LAD-I):
It is characterized by the inability of individuals to express β2 subunit (CD18) which is common to leukointegrins: LFA-1, Mac-1, p150/95 and αDβ2. As discussed previously in the chapter, leukointegrins are heterodimers consisting of α and β subunits. The α subunits are 4 in number (CD11a, CD11b, CD11c, and CD11d) whereas β subunit is common. Hence, it results in a defective adhesion of leukocytes.
- LFA-1: It is important for neutrophil diapedesis and lymphocytes scanning of antigen-presenting cells.
- MAC-1: It is important for adhesion of leukocytes to endothelial cells
- MAC-1 and p150/95: Both of them are important for complement receptors involved in phagocytosis
- αDβ2: It binds to ICAM3 and is distributed to certain macrophages and lymphocytes.
These defects occur in homozygous and heterozygous forms. Homozygous form manifests as generalized severe periodontitis whereas heterozygous may have normal prepubertal status. Histologically, LAD-1 shows dense plasma cell infiltration of periodontal lesions with copious immunoglobulin production (which appear histologically as Russell bodies).
Clinical manifestations:
The prominent clinical features of patients with LAD-1 are recurrent bacterial infections, primarily localized to skin and mucosal surfaces. The absence of pus formation at the sites of infection is one of the hallmarks of LAD-1. At birth these patients commonly present with infection, omphalitis (inflammation of the umbilical cord stump in the neonatal newborn period) with delayed separation of the umbilical cord. Severe gingivitis and periodontitis are the major features among all patients who survive infancy. Impaired healing of traumatic or surgical wounds is also characteristic of this syndrome 98.
Leukocyte adhesion deficiency type-II (LAD-II):
In this disease, leukocytes fail to express the natural ligands for P- and E-selectins which are sialo-Lewis X, gp150-Lewis X (CD15), and CLA (an epithelial antigen). Leukocytes have difficulty in adhering to the endothelial surface in response to inflammation. Patients with this defect show pronounced neutrophilia and recurrent bacterial infection at an early stage of life. LAD-II is associated with severe periodontitis. Neutro-philia (10,000-40,000/mm3) is a constant finding 105.
Clinical manifestations:
In this disease, affected children are born after uneventful pregnancies with normal height and weight. No delay in the separation of the umbilical cord is observed. Later on patients present with severe mental retardation, short stature, and a distinctive facial appearance. There is no pus formation at the site of infection. After the age of 3 years, the frequency of infections decreases and children no longer need prophylactic antibiotics 106.
Leukocyte adhesion deficiency type-III (LAD-III):
The precise molecular defect in LAD-III is still unknown and it may be the result of several different genes involved in the inside-out signaling for general integrin activation 107-109. Present evidence suggests that defects in the activation of β1, β2, and β3 integrin subunits result in an abnormal neutrophil function.
Clinical manifestations:
In few case reports described, the clinical presentation is very similar to LAD-I but it also includes defect in platelet activation 110 and a severe bleeding tendency 111.
Neutrophil function in periodontitis
The research work done on neutrophil functions in periodontitis cases, particularly in grade C periodontitis (aggressive periodontitis) cases suggests neutrophilic dys-function as one of the etiological factors associated with disease progression 112-114. The impaired chemotactic response of neutrophils in aggressive periodontitis patients has been proposed as one of the neutrophilic dysfunction 115. Hyperactive / “primed” neutrophils have been the focus of research for last few decades and have been proposed to be responsible for the rapid soft tissue destruction in aggressive periodontal diseases. Ryder (2010) 116 has proposed three possible mechanisms to explain the role of neutrophils in periodontal disease development. These are:
- The impaired neutrophil function.
- The hyperactive neutrophil function.
A third mechanistic category could also explain the role of neutrophils in periodontal disease development:
- Chronic recruitment and activation of the normal neutrophils.
A detailed description of neutrophil function defects and their role in periodontitis has been given in “Periodontitis”.
Conclusion
Neutrophils make up the primary line of defense against infection and their normal function is essential to prevent and eliminate infection from the body. In the above discussion, various clinical conditions have been discussed where the normal neutrophil function is disturbed. It has been well established now that patients with a defective neutrophil number or function demonstrate rapid periodontal destruction as compared to patients with normal neutrophil functions. Hence, a thorough understanding of neutrophil structure and function is essential to understand the etiopathogenesis of periodontal diseases.
References
References are available in the hard-copy of the website.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Suggested reading

