Introduction to local drug delivery
Topically applied antimicrobial solutions as mouth rinses have been used for many years as an adjunct to mechanical plaque control. Since oral rinses and irrigation at the gingival margin do not reach subgingival areas on a predictable basis 1, 2, a subgingival drug delivery system was desirable. The concept of controlled release local delivery of therapeutic agents in the periodontal pocket was pioneered by Dr. Max Goodson 3, 4, who made the initial drug delivery device made up of hollow fibers of cellulose acetate filled with tetracycline. In the previous chapter, we discussed in detail various systemically applied chemotherapeutic agents, their pharmacological properties and their application in various periodontal infections. However, systemic drug delivery is associated with various drawbacks due to which the concept of local drug delivery (LDD) is becoming more and more popular. In the following paragraphs, we shall discuss in detail the concept of LDD and various chemotherapeutic agents which are presently used for this purpose.
Terminologies
There are various terminologies which have been used to denote the prolonged release of drug in the past. These terms include sustained-release, controlled-release, prolonged-release, timed-release, slow-release, sustained-action, prolonged-action, or extended-action 5. However, the most commonly used terminologies are sustained release and controlled release. These terminologies are used ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Rationale for using local drug delivery
Periodontal pockets are populated by various periodontal pathogens which are responsible for the periodontal destruction. These pathogens may not be sometimes accessible to mechanical debridement. The primary rationale for LDD is to place an antibiotic or antiseptic in direct contact with the root surface, so that pathogenic microorganisms that are not accessible to mechanical removal by hand or power-driven instruments, can be reduced or eliminated. By using this method of drug delivery, a high concentration (many folds more than the minimum inhibitory concentration) can be attained at a site for a sufficient duration of time.

Classification
The local drug delivery systems can be classified as follows,
Based on the application:
Personally applied (in patient home self-care)
A. Non-sustained subgingival drug delivery,
Home oral irrigation
Home oral irrigation jet tips
Traditional jet tips
Oral irrigation (water pick)
Soft cone rubber tips (pickpocket)
B. Sustained subgingival drug delivery
Professionally applied (in dental office)
A. Nonsustained subgingival drug delivery
Professional pocket irrigation
B. Sustained subgingival drug delivery
Controlled release devices
Hollow fibres
Dialysis tubing
Strips
Films
Based on the duration of medicament release:
Sustained release devices: Designed to provide drug delivery for less than 24 hours
Controlled release devices: Designed to provide drug release that at least exceeds 1 day or for at least 3 days following application.
Depending on degradability:
Non-degradable devices (first generation).
Degradable devices (second generation).
Ideal requirements of a local drug delivery system
1. The drug delivery system must deliver the drug to the base of the pocket.
2. The delivery system must deliver the drug at a desired concentration, which is effective in killing micro-organisms.
3. The delivery system must sustain the concentration of the drug in the pocket for a sufficient duration of time.
4. It should be easily placed and manipulable.
5. It must be retained in place after initial placement.
6. It should be biodegradable.
7. It should not give rise to bacterial resistance.
8. It should be safe to use with minimal side effects.
9. It should be effective against periodontal pathogens only and not on commensal microflora.
Advantages and disadvantages associated with local drug delivery
There are many benefits as well as limitations associated with the used of locally delivered antimicrobial agents. These are enumerated as follows,
Advantages:
1. A high concentration (up to 100 fold higher as compared to systemic therapy) of the drug can be achieved at a localized site that can be maintained there, long enough for the desired effect to be accomplished without causing any side effect.
2. The concentration of the drug in periodontal pocket is not affected by the fluctuation in plasma levels.
3. The technique is suitable for agents which cannot be given systemically, such as chlorhexidine.
4. Superinfection and drug resistance are rare.
5. Reduction in total drug usage when compared with systemic therapy.
6. Reduction in drug accumulation with chronic therapy.
7. Reduction in frequency of drug administration
8. Improved patient compliance as the agent is placed by a dental professional
Disadvantages:
1. Most of the presently available antimicrobial agents for LDD can only be applied by dental professionals, which is time-consuming and requires special effort.
2. These agents have a limited action on periodontal pathogens residing within adjacent gingival connective tissues and on extra pocket oral surfaces (tongue, tonsils, and buccal mucosa), which increases the risk of later re-infection.
3. The drug may not get complete access to areas such as deeper pocket areas or furcations.
Indications of LDD:
Research done on the LDD in periodontal treatment has provided the evidence regarding its limited but beneficial use during active periodontal treatment and maintenance therapy. LDD is in no way a replacement of scaling and root planing, but is an adjunct when conventional therapy does not resolve the problem. Studies have compared monotherapy of LDD (used without mechanical instrumentation) agents with scaling and root planing and have demonstrated that there were no statistically significant difference between therapies relative to decreasing probing depth and gain of clinical attachment 8-13, so these must be used as an adjunct to scaling and root planing and not their replacement.
The therapy is primarily useful in following conditions,
- Patients with Grade A/B periodontitis where the initial treatment has been done, but certain sites are non-responsive to the treatment or the sites where surgery cannot be done (periodontal pockets at the distofacial line-angle of the last molar where surgical intervention is expected to yield a compromised result).
- Patients on maintenance, who are otherwise stable, but exhibit localized, persistent or recurrent deep pockets.
- Ailing/failing dental implants (peri-implantitis) where surgical intervention is not indicated or will yield a compromised result.
- Grade II furcation involvements (shallow or deep) when surgical intervention is not planned.
If the patient has multiple deep pockets and is demonstrating active periodontal destruction, surgical therapy is indicated. However, if the patient has a few pockets and a stable periodontal status, LDD along with scaling and root planing may be attempted after re-evaluation of the initial therapy. However, if the periodontal pockets still persist ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Contraindications of LDD:
It must be remembered that LDD is not a replacement for the traditional periodontal therapy. LDD should not be used in the following conditions,
- Patients with known hypersensitivity to any component of the LDD system to be used.
- As a replacement to scaling and root planing during initial periodontal therapy or maintenance.
- As a replacement for surgical periodontal therapy in cases indicated for periodontal surgery.
- In cases of Grade C periodontitis as there is insufficient data to support the use of LDD in these cases.
- As a replacement for systemic antibiotic therapy, where their systemic administration is indicated.
- Patients susceptible to infective endocarditis to avoid the risk of bacteremia.
Pharmacokinetics of chemotherapeutic agents in gingival sulcus
The gingival crevice is always filled with gingival crevicular fluid (GCF) which is constantly replaced by new GCF formed at the base of the gingival sulcus. Hence, it is obvious that if a chemotherapeutic agent is placed in a periodontal pocket, it shall be flushed out with time due to continuous flow and renewal of GCF. In a study, it was demonstrated that fluid present in the gingival crevice in a 5 mm periodontal pocket is replaced about 40 times an hour 7. This high rate of fluid clearance is attributed to a low resting volume (0.5 µl) and a comparatively high flow rate (20 µl) of GCF. The expected elimination half-time for a chemotherapeutic agent placed in the gingival sulcus is around one minute, which limits its efficacy in subgingival environment. Some chemotherapeutic agents have the property of ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Subgingival irrigation
Subgingival irrigation has been used for many years as a means for LDD in subgingival areas. As already stated, mouth rinses may not provide the complete access of an antimicrobial agent to the entire depth of periodontal pockets. So, subgingival irrigation with a needle or a nozzle that is placed at the entrance of gingival sulcus is helpful in pushing the irrigant till the base of the pocket. Presently, many professionally used and self-used devices are available, which have an inbuilt pumping system that produces the irrigant solution jet with adequate pressure in a continuous or pulsed manner. Various agents such as chlorhexidine (CHX), povidone iodine, tetracyclines, sodium hypochlorite and hydrogen peroxide have been used for this purpose.
The problem associated with subgingival irrigation is the quick elimination of the therapeutic agent from the gingival sulcus, which is due to the continuous renewal of GCF in gingival sulcus. However, CHX and tetracycline have the property of getting adsorbed on the surface and then released slowly with time (substantivity). However, the property of substantivity may not help in the maintenance of adequate concentration of the chemotherapeutic agents in the gingival sulcus. Therefore, a drug delivery system that provides continuous drug release in the gingival sulcus is desirable.
Local drug delivery carrier/vehicle systems
Presently, there are various drug carrier systems available to carry the active anti-microbial agents. These include,
- Fibers.
- Films.
- Injectable systems.
- Microspheres.
- Gels.
- Strips and compacts.
- Vesicular systems.
- Nanoparticle system.
Fibers:
The fiber system consists of thread-like devices which act as a reservoir for the active drug. These fibers are placed circumferentially into the pockets with an applicator and secured with cyanoacrylate adhesive. While secured in place, these fibers release active drug for an extended period of time. Various materials have been used to make these fibers including poly (e-caprolactone) (PCL), polyurethane, polypropylene, cellulose acetate propionate and ethyl vinyl acetate (EVA). The examples of commercially available systems using fibers include tetracycline fibers and chlorhexidine fibers.
Films:
Films used as a carrier for active drugs are made up of synthetic biodegradable polymers such as poly (lactide-co-glycolide) (PLGA) or cross-linked fish gelatin (Byco protein). The synthetic films may be prepared either by solvent casting or direct milling. These are cut into small pieces suitable for insertion into periodontal pockets. The drug is released slowly with the degradation of carrier matrix. These can be easily placed in the periodontal pocket and no additional aids for retention are required because of the adhesive nature of the carrier material. The film, based LDD systems are available for chlorhexidine diacetate, metronidazole, tetracycline, and minocycline.
Injectable systems
These are comparatively easy systems for delivering the antimicrobial agent in the periodontal pocket. The drug can be easily delivered without pain. The drug is pushed into the periodontal pocket so that it can reach the deepest portion of the pocket thus getting access to the microflora of periodontal pocket.
Microspheres:
This system uses biodegradable material to make microspheres containing the active drug, which is released by the degradation of the microspheres. The rate of degradation of the polymer used to make microspheres determines the rate of drug release. Microspheres can be made up of synthetic polymers or natural polymers. Synthetic polymer microspheres are usually made up of biodegradable polymers, e.g., lactides, glycolides and their copolymers, poly alkyl cyanoacrylates and poly anhydrides. Natural polymers are obtained from different sources like proteins (albumin, gelatin, and collagen), carbohydrates (agarose, carrageenan, chitosan and starch) and chemically modified carbohydrates (poly dextran and poly starch). Depending on the rate of hydrolysis of ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Gels:
Various gel formulations are also available for LDD. These systems are based on hydroxyethyl cellulose, corbopol 974, and polycarbophil. The gel carrying active drug is applied in the periodontal pocket with the help of a blunt syringe. A newer development in gel technology is that of hydrogels. These are polymeric materials that do not dissolve in water at physiological conditions. However, they swell considerably in aqueous medium 16. These gels have a phase transition in response to the changes in external conditions such as pH, ionic strength, and temperature which make them degradable 17. High water content and large pore sizes of most of the hydrogels often result in the relatively rapid drug release, over a few hours to a few days. The examples of presently available gel based LDD systems include metronidazole gel and doxycycline gel.
Strips and compacts:
Strips are made from mixing polymers, monomers and different concentrations of the chemotherapeutic agents. The strips are flexible for easy placement and have a position securing mechanism. Similar to films, these are placed in the periodontal pocket where with progressive degradation of the polymer, the drug is released into the surrounding environment, maintaining a high level of chemotherapeutic agent. Strips containing tetracycline, metronidazole or chlorhexidine have been tested for their efficacy in the reduction of periodontal pathogens.
Vesicular systems:
These are primarily liposomal systems similar to bio-membranes in terms of structure and bio-behavior. Structurally, liposomes are concentric bilayer vesicles in which an aqueous volume is entirely enclosed by a membranous lipid bilayer, mainly composed of natural or synthetic phospholipids. Liposomes have the distinct advantage of being both non-toxic and biodegradable because they are composed of naturally occurring substances. Chemotherapeutic ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Nanoparticle system:
The most recent introduction in the field of targeted drug delivery is nanoparticle system. Various types of nanoparticle systems have been designed for LDD, including bio-degradable polymeric nanoparticles, polymeric micelles, nanocapsules, nano-gels, fullerenes, solid lipid nanoparticles (SLN), nanoliposomes, dendrimers, metal nanoparticles and quantum dots. The main advantages of these systems are high dispersibility in an aqueous medium, controlled release rate, and increased stability. Due to their small size, these particles can penetrate the regions that may be inaccessible to other delivery systems, such as deep periodontal pockets and furcation areas. A lot of research is going on in this field and in future commercially available nanoparticle-based LDD systems are expected.
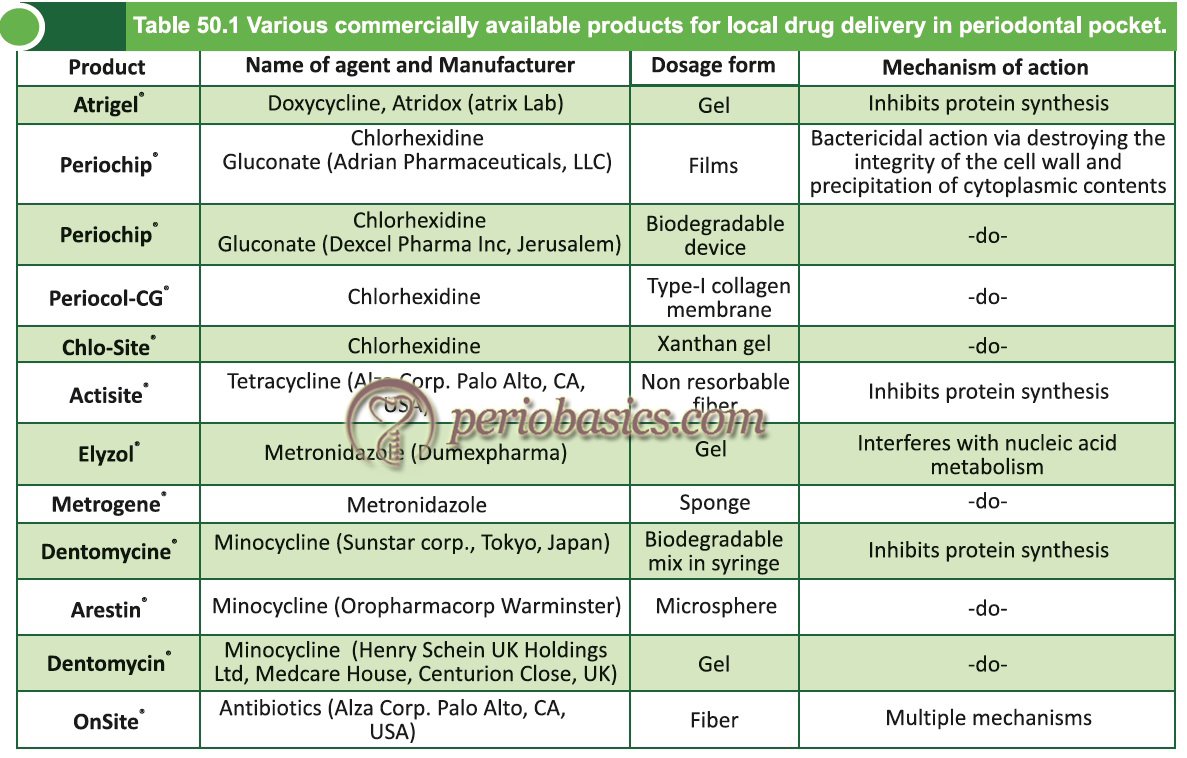
Commercially available local drug delivery systems
Tetracycline fibers (Actisite®):
The LDD system for tetracycline was developed initially by Goodson in 1979 3 using a small diameter cellulose acetate tubing system. The drug is diffused into the periodontal pocket by osmotic pressure. The drug delivery system used to degrade in approximately six hours. Due to poor control of drug release from hollow fibers, Goodson evaluated other material. Ethylene vinyl acetate (EVA) was found to be flexible and release the drug for up to 9 days 20. Since then, research has been going on in the development of a carrier system to deliver tetracycline in a controlled manner in the periodontal pocket. The result was tetracycline fiber system Actisite®. The system consists of non-resorbable fibers made up on biologically inert plastic copolymer loaded with 25% of ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……

Various studies have been done to find the effectiveness of tetracycline fibers in the improvement of patient’s periodontal status. In an intra-individualized multi-centric randomized controlled trial, Goodson et al. (1991) 21 evaluated the short-term effectiveness of tetracycline fibers. A comparative evaluation was done to find out the improvement in clinical parameters after scaling and root planing (SRP) (positive control), untreated sites (negative control), tetracycline fibers placement and unloaded EVA fibers placement (vehicle control). All the sites received supragingival scaling and each treatment was applied to one tooth in the dentition. The parameters assessed included probing depth, clinical attachment levels and bleeding on probing. The results demonstrated improvement in all parameters as compared to controls with tetracycline demonstrating only a limited response in the improvement of clinical parameters assessed.

In another study, Newman et al. (1994) 22 compared SRP and the combination of SRP + LDD with tetracycline fibers. In this study, those patients were included in which there were persistent pockets which did not respond to the initial treatment. The results of the study demonstrated a significant improvement in the clinical parameters including probing depth, clinical attachment levels and bleeding on probing in areas with combined therapy (SRP + LDD with tetracycline fibers) as compared to monotherapy with SRP.
In a large-scale study, 23 four treatment modalities were compared. These included SRP, SRP+LDD with tetracycline fibers, two sequential applications of SRP and SRP+LDD with tetracycline fibers. Three quadrants were treated with experimental treatment and the fourth quadrant was treated by SRP. In a 12 month observation period, no significant difference was found in four treatment modalities.
Radvar et al. (1996) 24 compared the efficacy of different LDD agents in improving the clinical parameters in sites with persistent periodontal pockets. They compared SRP, SRP+ tetracycline fibers, SRP+ metronidazole gel and SRP+ minocycline ointment application. There was a significant improvement in clinical parameters in all the ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Doxycycline gel (Atridox®):
As discussed in the previous chapter, doxycycline has a wide spectrum of activity against periodontal pathogens. Atridox® is FDA approved 10% doxycycline in a gel system containing 42.5 mg doxycycline. Its formulation for LDD in gel form has been made, named Atridox®. It is composed of a polymer gel carrier which flows into the gingival sulcus and transforms into wax like consistency when it comes in contact with GCF. The delivery system consists of two syringes. One syringe contains the liquid delivery system and the other syringe contains drug powder. The procedure of drug delivery involves uncoupling the two syringes after mixing, attaching a blunt cannula to one syringe and placing the gel in the periodontal pocket by inserting the tip of the cannula slowly into the periodontal pocket. The drug release from the carrier (vehicle) takes place within seven days. It is recommended that after placement of the gel, the area should be covered with a protective dressing, such as cyanoacrylate dressing. Around 95% of the polymer is bio-absorbed or expelled from the pocket naturally within 28 days 28.
In a study Polson et al. (1997) 28 compared the efficacy of doxycycline, sanguinarine chloride and a vehicle control in the improvement of clinical parameters (probing depth, clinical attachment levels, bleeding on probing and plaque index) in periodontitis patients who had received surgical or non-surgical periodontal therapy six months prior to the study. The results of the study revealed a significant improvement in the clinical parameters with doxycycline as compared to sanguinarine chloride and a vehicle control. The authors suggested the use of doxycycline LDD as an effective and safe treatment for periodontitis. In another study done on smokers with periodontitis, Machion et al. (2004) 29 found significantly greater probing depth reduction among smokers who were administered SRP plus doxycycline gel.
Garrett, et al. (1997) 30 in two randomized, single-blind, multi-centric trials compared the efficacy of SRP, doxycycline, vehicle control and oral hygiene in improvement of clinical parameters which included probing depth, clinical attachment levels, bleeding on probing and plaque index. Each study was done over a period of nine months and the ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Chlorhexidine:
There are many commercially available LDD systems containing chlorhexidine (CHX). Following is the description of three commonly used CHX-containing LDD systems.
PerioChip®:
CHX has been used widely as plaque control measure in the form of mouth rinses. It has a proven efficacy in preventing plaque and gingivitis because of which it is considered as the gold standard of oral plaque control. However, as already stated it may not reach the complete depth of periodontal pocket when used as a mouth rinse. In 1990’s a LDD formulation containing CHX was developed which was named PerioChip®. The system consisted of bio-absorbable gelatin matrix containing 2.5 mg of chlorhexidine gluconate. Each chip measures 4 mm x 5 mm x 0.35 mm. The strip is orange-brown in color ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Periocol-CG®:
This system consists of Type-I collagen membrane (derived from fish sources) containing 2.5 mg CHX derived from a 20% CHX solution. The chip weighs 10 mg and size of the chip is 4mm x 5 mm with a thickness of 0.25 – 0.32 mm. Collagen membrane which has been used in this system has many advantages. It is naturally occurring protein having excellent hemostatic properties. It is chemotactic for fibroblasts, enhances fibroblast attachment via its scaffold-like fibrillar structure. 40-45% of CHX is released within the first 24 hours of placement and the remaining drug is released in a linear fashion within 7-8 days. The chip completely resorbs within 30 days.
Chlo-Site®:
It is a gel-based system that contains 1.5% CHX. The carrier system is made up of Xanthan gel which is a saccharidic polymer that when combined with water forms a three-dimensional pseudo-plastic reticulum. This matrix is capable of retaining chemotherapeutic agents which are then released slowly depending on their physical and chemical characteristics. The gel has good adhesive properties in the periodontal pocket and does not require periodontal dressing. The gel gets dissolved within 10-30 days after placement. The effective antimicrobial concentration of CHX is maintained for up to 15 days.
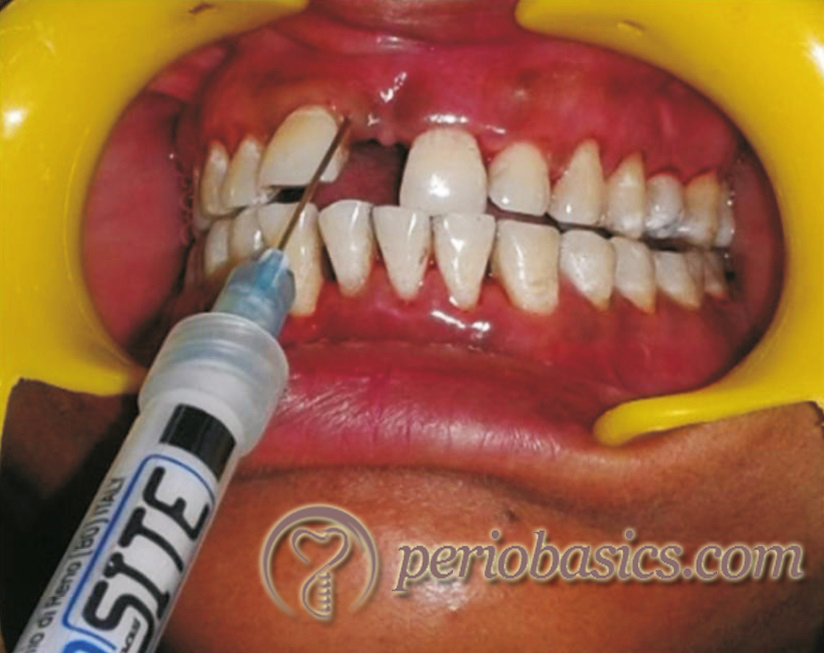
Studies have been done to evaluate the reduction in periodontal pathogens after subgingival placement of chlorhexidine LDD systems. Stabholz et al. (1986) 31 placed ethyl cellulose strips with CHX in periodontal pockets every 3 days for a total exposure of 9 days. The results of the study reported a decrease in spirochetes and motile rods and reduction in pocket depth for up to 11 weeks following the therapy.
Many studies have been done to analyze the changes in clinical parameters following LDD with CHX. In a 9 month clinical trial, Jeffcoat et al. (2000) 32 evaluated the changes in clinical parameters after SRP, CHX chips + SRP and placebo chip + SRP in periodontitis patient. The results of the study demonstrated that 15% of the sites where only SRP alone was done lost bone (0.04 mm). Whereas, sites with SRP+ CHX chips, achieved a mean 0.1 mm gain of bone. In another study done on smokers, Soskolne et al. (1997) 33 demonstrated better results with SRP+ CHX chip than only SRP among patients with chronic periodontitis.
Minocycline:
Minocycline belongs to tetracycline group and is a bacteriostatic antibiotic. It’s preparation for LDD has been tried clinically in three different modes: film, microspheres and ointment 34. Arestin® is microsphere-based and Dentomycin® is ointment based commercially available LDD systems for minocycline.
Arestin®:
In this system, microspheres containing minocycline are used. The microspheres are 20-60 µm in diameter. One milligram of minocycline is microencapsulated into a bio-absorbable polymer and delivered subgingivally as a powder via a syringe. Once these microspheres come in contact with GCF, they release the drug at a slower rate, but sufficiently above the minimum inhibitory concentration (MIC), over a period of at least 14 days. The released drug effectively reduces the number of microorganisms in periodontal pocket.
Dentomycin®:
The gel vehicle for LDD of minocycline has been developed which consists of a matrix of magnesium chloride hexahydrate, ammonio methacrylate copolymer, triacetin, and glycerol. The gel contains 2% minocycline. The delivery system is provided ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Studies evaluating the reduction in periodontal pathogens after application of minocycline have been performed. In one investigation 30% minocycline was used in ethyl cellulose film. The results of the study demonstrated a significant reduction in pathogenic microflora 35. However, increased resistance of microorganisms has also been reported with use of minocycline and other agents. It has been demonstrated that exposure to subinhibitory concentrations of metronidazole or minocycline, resulted in the development of resistance among P. gingivalis, P. intermedia, F. nucleatum, and P. anaerobius 36. In another study Preus et al. (1995) 37 also reported a temporary increase in the resistant microorganisms, after local application of minocycline ointment.
Several clinical trials have evaluated the effect of applying 2% minocycline gel after root planing, on the improvement of clinical parameters. In one study, Steenberghe et al. (1993) 38 reported a significant greater probing depth reduction at sites with pocket depth ≥7 mm when combined therapy (SRP+LDD) was delivered as compared to SRP. Whereas, Timmermans et al. (1996) 39 found no significant differences in gain in attachment levels and Graca et al. (1997) 40 reported only 0.3 mm of attachment gain after combined therapy with SRP+ 2% minocycline gel application.
Metronidazole:
Metronidazole is effective against various Gram-negative microorganisms and is primarily used for the treatment of anaerobic periodontal infections. Its formulations for LDD have been developed. The commonly used LDD preparation containing metronidazole includes Elyzol® and Metrogene®.
Elyzol®:
It consists of a bioabsorbable gel that contains 25% metronidazole benzoate in a sesame oil matrix. The gel is placed in the periodontal pocket with a syringe and a blunt cannula. The gel in periodontal pocket liquidizes due to body heat and then again solidifies due to contact with water to form crystals. It is available in 1 gm and 0.3 gm preparations. 1 gm preparation contains 250 mg metronidazole and 0.3 gm preparation contains 75 mg of metronidazole. The activity and resorption of the material occur within 12-24 hours. Re-application of the gel is done after 7 days.
Metrogene®:
The system consists of 5% metronidazole in natural bovine collagen. The material is supplied in the form of sponge square pieces. The sponge is placed in the periodontal pocket and when it comes in contact with GCF, it rapidly forms a resorbable gel which releases the drug in a slow manner.
The microbiological investigations done on metronida-zole gel have demonstrated a marginal effect with respect to decrease in the total anaerobic bacteria colony forming units in the subgingival plaque 12, 41. This may be attributed to a low number of bacteria responsive to metronidazole and loss of the substantial amount of drug due to swallowing or absorption through the mucosa. Studies done to evaluate the clinical improvement in periodontal parameters with metronidazole gel when compared with positive treatment control, consisting of SRP did not come up with much significant findings. In general, no significant difference was found between different therapies in the reduction of probing depth and prevalence of bleeding on probing 10, 11, 13, 25. However, a few studies comparing SRP to combined therapy with SRP+ metronidazole gel have reported statistically significant improvement in clinical parameters with the combined therapy 42, 43.
Future trends in local drug delivery
A lot of research work is going on in the field of LDD in treating periodontitis patients. A major goal of this research is to attain a sufficiently high levels of the anti-microbial agents in the periodontal pocket for a sufficient duration of time to achieve desired results and at the same time evoking minimal or no side effects. Newer chemotherapeutic agents have been examined for their effectiveness in periodontitis cases. Clarithromycin gel 44 has recently been examined for LDD in chronic periodontitis cases and has been found to be marginally beneficial. Various newer vehicles to carry the chemotherapeutic agents have also been investigated. PT-01, a subgingival delivery system containing ofloxacin was found to be effective in bleeding on probing and reduction in plaque score 45.
Similarly, colloidal drug carriers including micelles, emulsions, liposomes and nanoparticles are being investi-gated for LDD. Dung et al. (2007) 46 prepared antisense oligonucleotide loaded chitosan nanoparticles and investigated the release of the oligonucleotide from chitosan-TPP/oligonucleotide nanoparticles . The results of the study showed that release of oligonucleotides from chitosan/ oligonucleotide nanoparticles was stable enough for 12 hours under the 20% saliva solution. The authors suggested the delivery system useful for sustained release of chemo-therapeutic agents in the treatment of periodontal diseases. Pinon et al. (2005) 47 used ……. Content available in the book ……. Content available in the book …… Content available in the book ……. Content available in the book ……
Conclusion
The present literature on LDD suggests that it is a beneficial treatment in conjunction with routine SRP, but not as a monotherapy. Furthermore, adjunctive treatment with LDD may provide a definite but limited improvement in the periodontal status of the patient. So, clinicians need to assess the cost-benefit ratio before going for this treatment and to assess alternative therapies which may provide definite and better results as compared to LDD. A lot of research work is being done presently on various chemotherapeutic agents to be used as LDD agents. In near future it is anticipated that newer agents which are more effective in halting the periodontal disease progression will be introduced.
References
References are available in the hard-copy of the website.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.

