Introduction to humoral immunity
Humoral immunity is the type of host defense that is mediated by antibodies, the products of B-cells. Antibodies are secreted into mucosal lumens, blood, and interstitial fluids, and combat microbes at all these sites. As already stated, B-cells are the source of antibodies, let us first understand the development and maturation of B-cells. During embryo-genesis, B-cells are first recognized in the liver. From there they migrate to the bone marrow, they don’t require thymus 5.
Origin and development of B-lymphocytes
B-lymphocytes are generated from the common lymphoid progenitors, which are originated from the differentiation of the hematopoietic stem cells. The yolk sac, fetal liver, and adult liver are the sites in the body where these cells are generated and developed 17-19. B-lymphocytes play crucial roles in host defense against infection via a series of highly coordinated processes that include cell homing, antigen recognition, antibody secretion, antigen presentation, and/or cytokine release. The steps of maturation of B-cells are as follows:
- In the bone marrow in early stages, the B-cell precursors (pre-pro B-cells) must interact physically with the stromal cells for their proliferation and maturation to occur. Later stages (late pro B-cells) merely need the soluble growth factors produced by stromal cells. Stromal cells produce several necessary growth factors and cell-cell adhesion molecules. One key growth factor for B-lymphopoiesis is interleukin-7 (IL-7).
- The earliest identifiable stage of B-cell differentiation is the pre-pro B-cell. At this stage, the process of immunoglobulin gene rearrangement begins. The first re-arrangement entails the joining of the D segment to the J segment of the immunoglobulin heavy chain gene (IgH). Subsequent rearrangements bring the V region juxtaposed to the DJ portion. After this, surrogate light chains can combine with the μ heavy chain protein in pro- and pre- B-cells, forming a structure referred to as the pre B-cell receptor.
- A newly formed B-cell displays IgM on its cell surface. At this stage, the B-cell is still immature and responds to antigen differently than a mature B-cell. Immature B-cells can be functionally removed by interaction with self-antigen, either by undergoing programmed cell death (apoptosis) or by anergy, in which the cell is rendered non-responsive in the presence of the antigen. Thus, similar to T-cells, immature B-lymphocytes undergo a process of “negative selection” to delete cells that are reactive to “self” antigen.
- Immature B-cells that are not removed by the processes of negative selection leave the bone marrow and migrate to peripheral or secondary lymphoid tissues such as spleen and lymph nodes. Here, further maturation takes place and newly formed B-cells express IgD, in addition to IgM, on the cell surface. The mature B-cells are now fully responsive to antigens and interaction with T-cells and actively participate in the humoral immune response.
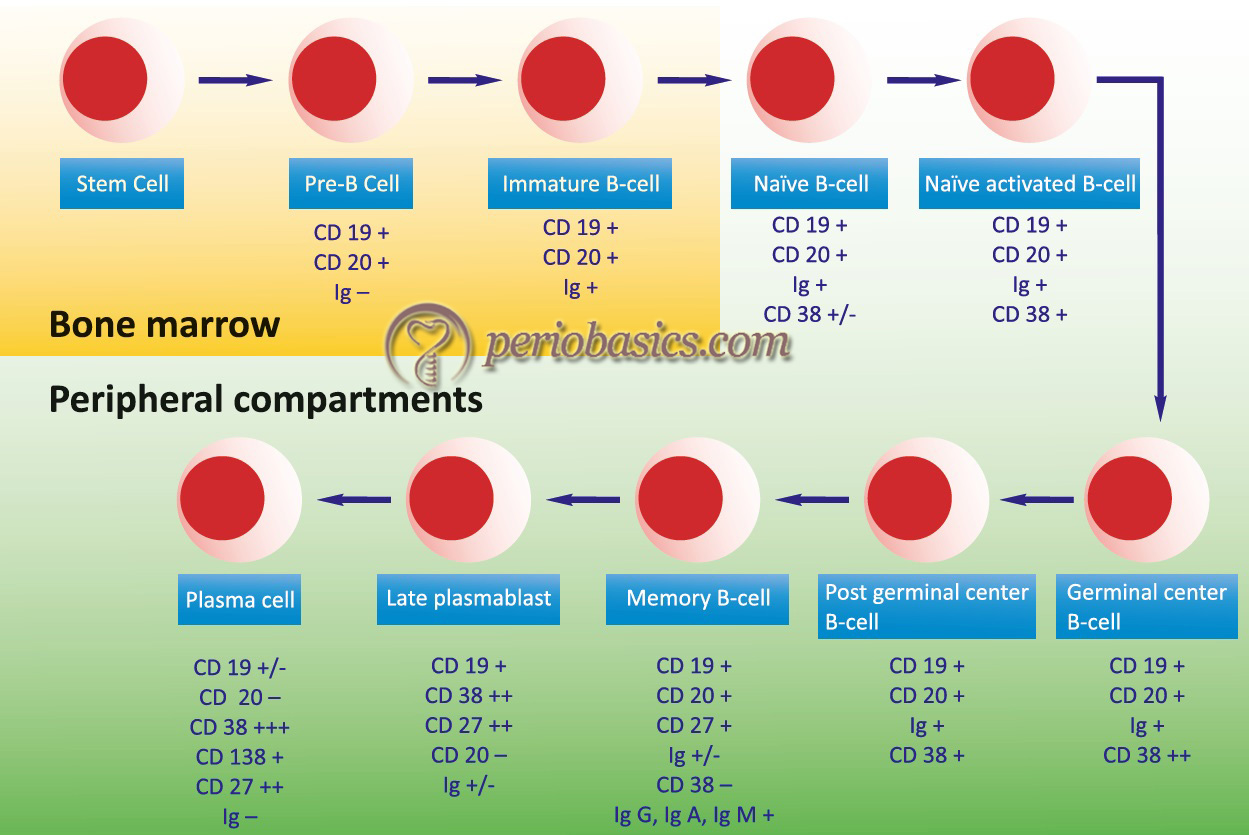
Phases of B-Cell development and maturation
The development and maturation of B-cells occur in multiple steps. The stages of B-cell development start with stem cells that differentiate into the pre-B-cell and then into mature B-cell (explained later in the chapter). During B-cell maturation, the first phase of maturation is an antigen-independent phase consisting of stem cells, pre-B-cells, and B-cells. The second phase of maturation is an antigen-dependent phase, which comes into play due to the interaction of antigen with B-cell. IgM (sometimes IgD) is first displayed on the B-cell surface, which acts as a receptor for antigens. B-cells constitute about 30% of the circulating lymphocytes 6. Their life span ranges from days to weeks. Approximately 109 B-cells are produced each day. There is a large pool of B-lymphocytes approximately 107 with different specificities.
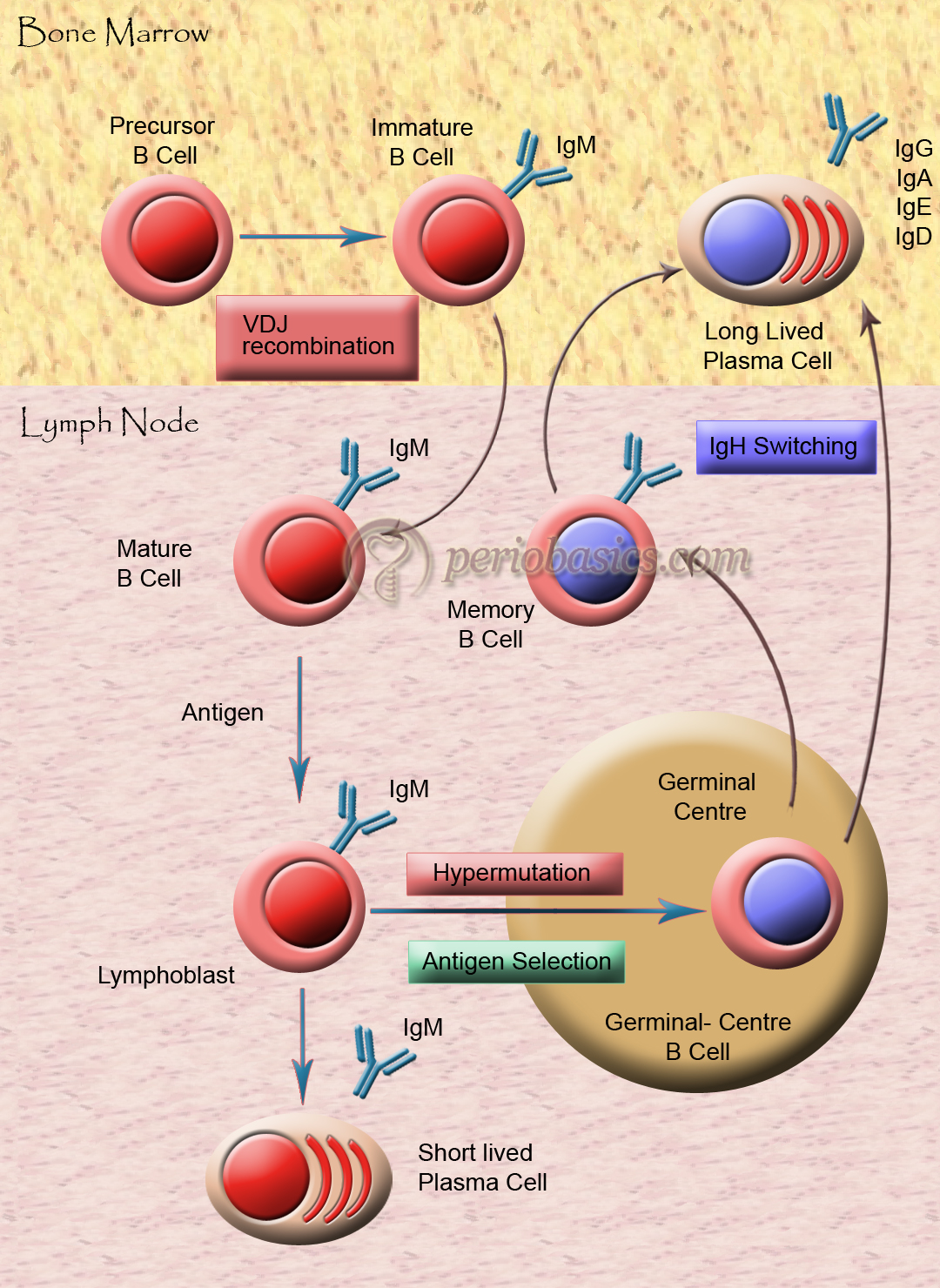
Activation of B-cells
Multivalent antigens directly attach to IgM and IgD on the B-cell surface. It is due to the cross-linkage of antigen with antibodies on the B-cell surface. Another way is via T-cells, through the secretion of IL-4 and IL-5. For the activation of T-cells, the co-stimulatory interaction between CD40-CD40L and B7-CD28 is necessary (discussed later).
Antibody structure and humoral response
Soluble proteins that circulate in the body and perform the major functions of the immune system are antibodies. Initially, when found, they were called γ globulins (as per their migration on the electrical field) but today they are called immunoglobulins. The two most important features of immunoglobulins are specificity and biological activity. These can identify a large number of antigens. One part of the antibody is adaptable to a large number of epitopes and the other part participates in biological activities 7.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Isolation of immunoglobulins:
Electrophoresis of serum gives five major components: albumin, α1, α2, β and γ globulins. Out of these, γ globulin is least migrating. It forms a very small peak so was difficult to identify. However, in the case of multiple myeloma, immunoglobulins are elevated, so this peak is particularly high. In 1845, Henry Bence Jones discovered immunoglobulin proteins in the urine of multiple myeloma patients. These proteins are known as Bence Jones proteins.
Structures of Light and Heavy Chains:
Porter in 1959 found that proteolytic treatment of enzyme papain splits immunoglobulins into three parts with almost equal molecular weight 8. Two of these parts can bind to antigen but no longer precipitate. These two parts are called fragment antigen-binding (Fab). The third fragment could be crystallized due to the property, indicative of its apparent homogenicity, so it was called as fragment crystallizable (Fc). It could not bind to antigen but it could participate in biological functions. His data led to the proposal of a Y-shaped structure of an antibody (Figure 6.4). At the same time, Edelman in the US discovered that γ globulins were extensively reduced by treatment with mercaptoethanol (an agent that breaks disulfide bonds). It broke immunoglobulin molecule into four chains, two heavy chains (H chains) of identical molecular weight up to 53,000 Daltons (D) and two light chains (L chains) having a molecular weight of 22,000 D 9-11. On the basis of these findings, they proposed a structure of Immunoglobulins and got a noble prize in 1972. In the due course of time, the structure of various antibodies was proposed.
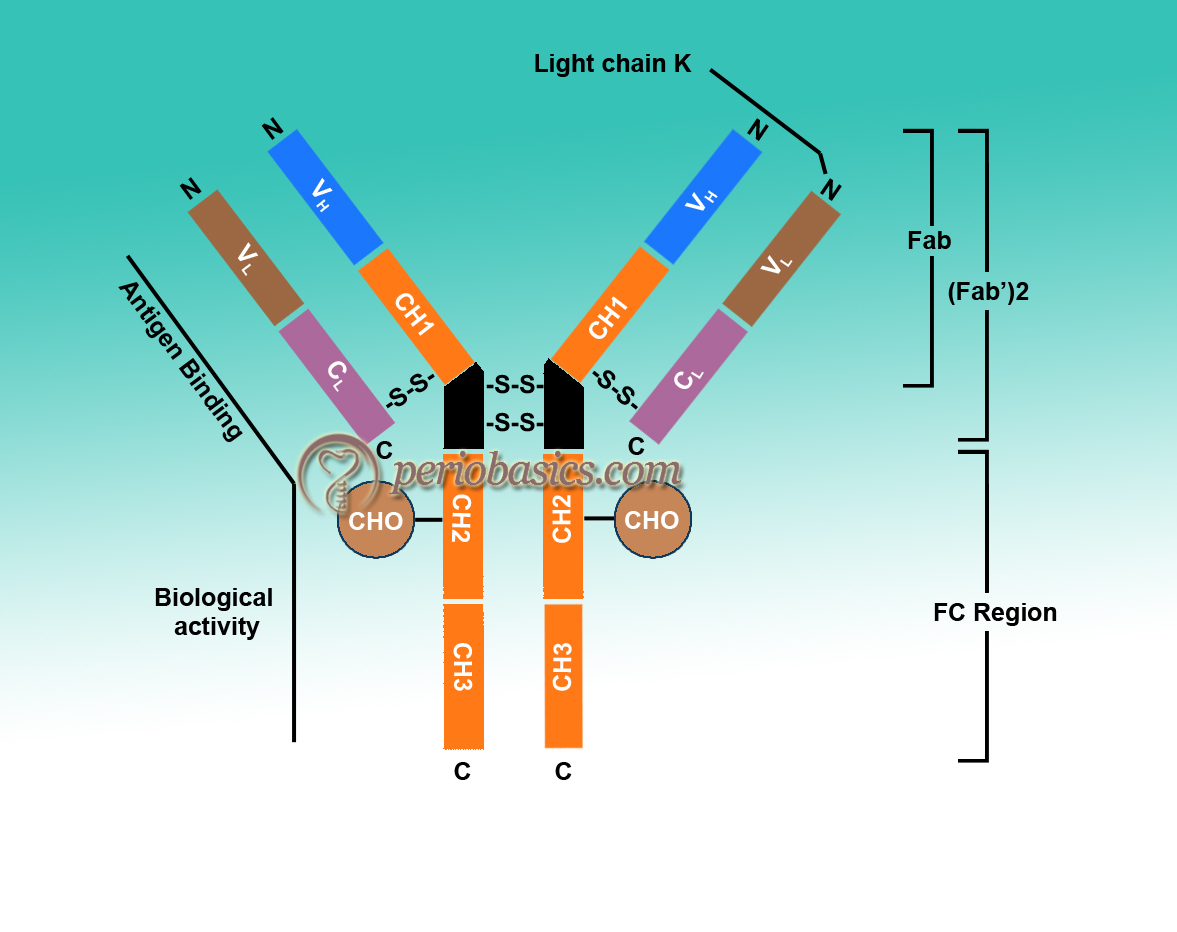
Basic structure of Immunoglobulins:
- As already stated, the structure of immunoglobulins consists of light and heavy chains. A light chain contains about 211 to 217 amino acids and a heavy chain contains approximately 450 amino acids.
- All the species have only two types of light chains, λ and κ. Every individual within a species synthesizes both of these chains, but ratio, i.e. λ/κ varies from species to species (in mouse 95% and in human 60% are κ chains). But in any immunoglobulin molecule, both light chains are the same (either λ or κ). In humans, the ratio of immunoglobulin containing κ chains to those containing λ chains is approximately 2:1.
- Heavy chains of virtually all species consist of five different classes (isotypes) that differ in their structure. H chains of different immunoglobulins are IgM-µ, IgG-γ, IgA-α, IgE-ϵ, IgD-δ. Two heavy chains in an immunoglobulin molecule are the same. For example, IgG molecule can have κ2γ2 or λ2γ2.
- Heavy chains confer on an immunoglobulin molecule its unique property such as half-life in circulation, its ability to bind to certain receptors and activate enzymes.
- Further subdivision of these molecules gives subclasses. For example, IgG has been divided into IgG1, IgG2, IgG3, IgG4. In the same way, IgA has been divided into IgA1 and IgA2. These sub-isotypes vary in number and arrangement of inter-chain disulfide bonds.
- L and H chains are subdivided into variable and constant regions. There are disulfide bonds present within a chain and between two chains. In a chain, these disulfide bonds make loops, called domains. Most of the H-chains contain one variable (VH) and three constant (CH) domains. IgG and IgA have three CH domains, whereas IgM and IgE have four CH domains. Each domain contains about 110 amino acids.
- VH and VL are responsible for antigen binding, whereas the constant region is responsible for various biological functions, such as complement fixation and binding to the cell surface receptors. Certain amino acids in hypervariable regions are very variable. Only 5 to 10 amino acids in each hypervariable region form the binding site. There are three hypervariable regions present in both VH and VL. If we count from the amino acid terminal, these are found in and around 30, 50 and 95 amino acid regions which are called complementary determining regions (CDR’S). Antigen-antibody binding involves electrostatic and van der Waals forces.
- Immunoglobulins of one species are antigenic to other species.
Individual immunoglobulins
Immunoglobulin G
It is the predominant immunoglobulin in the blood that makes about 75% of the total immunoglobulins. The molecular formula is H2L2. It is a monomer and is divalent (has two identical antigen-binding sites). It has got four subclasses with a ratio IgG1 : IgG2 : IgG3 : IgG4 = 66 : 23 : 7 : 4. It is synthesized in the fetus as early as 20 weeks of gestation and has a molecular weight of about 150000 D. Its sedimentation coefficient is 7S. Electrophoretically, the IgG molecule is least anodic and migrates to the gamma range. Except for the variable region, all immunoglobulins in one class have 90% homology. Thus, the antiserum is active against all classes of a particular immunoglobulin. The half-life of IgG1, IgG2, and IgG4 is 23 days, whereas for IgG3 it is 7days. IgG1 is responsible for erythroblastosis fetalis. The main functions of IgG include,
- Opsonization.
- Antibody-dependent cell-mediated cytotoxicity (ADCC): Virus-infected cells can be destroyed by a combination of IgG and phagocytic cells. The antibody binds to the surface of the infected cell. This antibody is recognized by its receptor present on the phagocytic cell (macropha-ges or NK cells). The infected cell is then killed.
- Activation of the complement system.
- Neutralization of toxins.
- Immobilization of bacteria by clumping their flagella and cilia.
- Neutralization of viruses (by attaching to the surface of the virus and preventing its attachment to target cell).
Immunoglobulin M
IgM is the first immunoglobulin to be produced after immunization. It is found in monomer or pentamer structure. For a pentameric structure, the molecular weight is 900000 D and has a sedimentation coefficient of 19s. Five units are joined to each other by disulfide bonds. A polypeptide chain joins the Fc portion of monomer units called as J chain. This J chain is synthesized in B-cell or plasma cells. It has a molecular weight of 15000 D. Valancy of IgM is 5 instead of 10, which is because of the congested structure of this immunoglobulin. Its Fab portion cannot open fully allowing attachment only to 5 antigen epitopes. If IgM is found to be raised in newborns, it is indicative of intrauterine infections of rubella, syphilis, toxoplasmosis, cytomegalovirus infections. It is an active activator of the complement system by classical pathway.
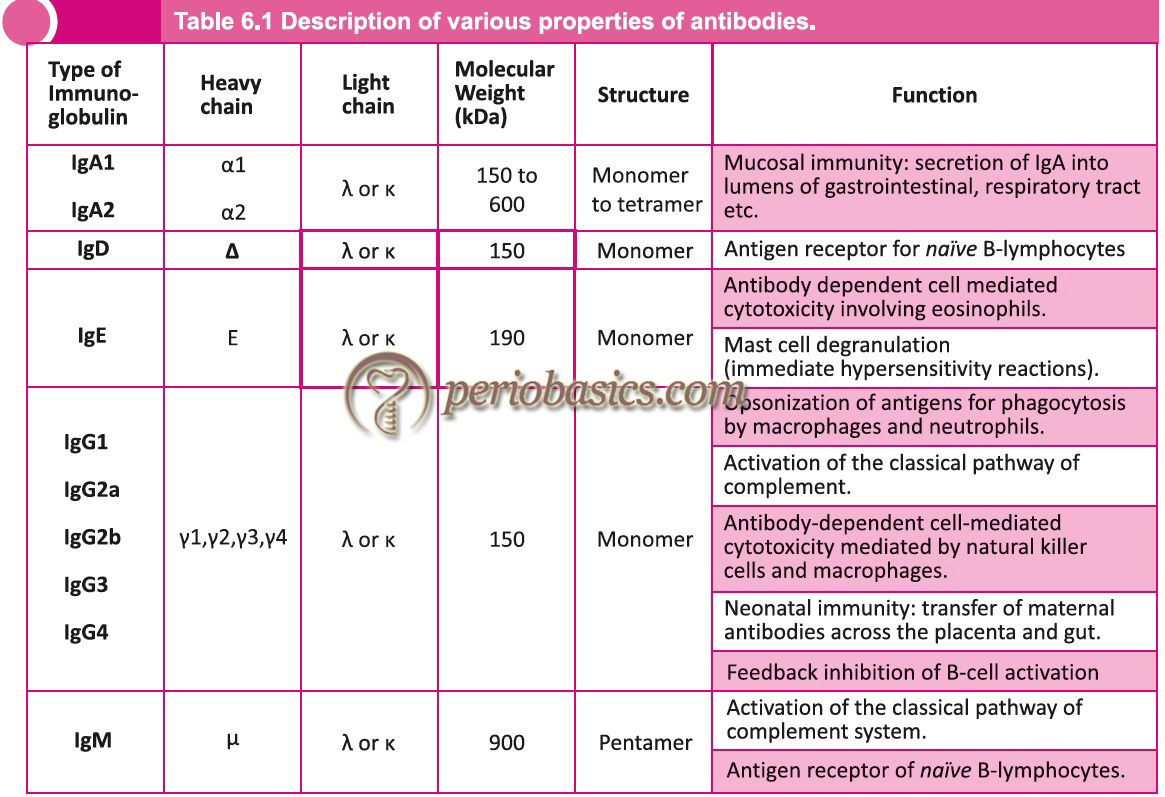
Immunoglobulin A
It is the major immunoglobulin present in the external secretions such as saliva, mucous, sweat, gastric fluid and milk. It provides the neonate with protection against intestinal infections. Its molecular weight is around 165000 D. Its sedimentation coefficient is 7s and it migrates to β or fast γ region. It may exist as a monomer or dimer. In secretions, it is secreted as a dimer. The dimers are united together by a J-chain covalently . IgA also has another polypeptide chain called the secretory component. The joining chain is a product of plasma cells. The secretory component is a product of epithelial cells and plays an active role in the transportation of IgA into various secretions.
Immunoglobulin D
IgD is present in serum in a very low concentration and its amount is variable. It is probably not secreted by plasma cells. It has not been designated for any definite function. But, it is said to be associated with IgM as a surface component of many B-cells. It is a monomer with a molecular weight around 150000 D and a sedimentation coefficient of 7S.
Immunoglobulin E
It has an extra CH domain. Structurally, it is a monomer with a molecular weight around 190000 D. Its sedimentation co-efficient is 8S and migrates to fast γ globulin region. IgE is called as “reaginic-antibody”. It is present in serum in the lowest concentration. It has a specific domain on its H chain which attaches to the mast cells with high affinity. It attaches to mast cells and basophils through its receptors in Fc region. There are many cell surface receptors for FcR1 (104 on basophils and 106 on mast cells). When two IgE molecules are cross-linked on these cells, they become activated and secrete their contents.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
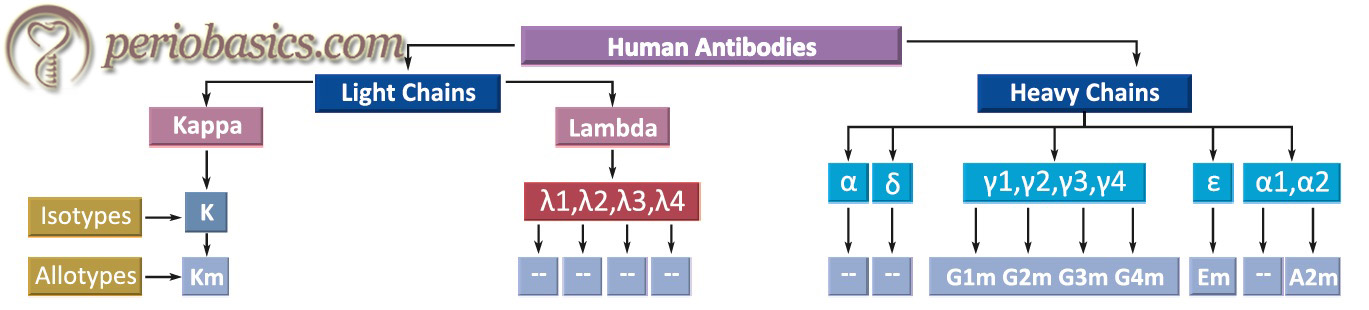
Light and heavy chains of human antibodies
Antibodies can also be explained in terms of isotypes, allotypes, and idiotypes.
Isotypes:
Isotypes of antibodies are formed due to the differences in the amino acid sequence in their constant region. For example, IgG and IgA are isotypes as their heavy chains are different antigenically. Individual immunoglobulins can have their own subtypes, for example, IgG has its subtypes IgG1, IgG2, IgG3, and IgG4 (based on the antigenic difference on their heavy chains).
Allotypes:
Some features of antibodies vary from person to person, for example, IgG has 2H and 2L chains. Genes that code for γ chain are polymorphic. Every individual is inherited with different alleles which change the amino acid sequence from person to person.
Idiotypes:
These are antigenic determinants formed by specific amino acids present in the hypervariable region. Each idiotype is unique for an antibody-producing cell. Hence, it can be summarized that,
- The constant region of the heavy chain determines the immunoglobulin class.
- The constant region of heavy and light chains determine the allotypes.
- The variable region of light and heavy chains determine idiotypes.
- The constant region of the heavy chains binds IgG to macrophages.
- The fixation of complement is also in the constant region of the heavy chains.
- Variable regions of light and heavy chains make the antigen-binding sites.
Know more…
Some facts about antibodies:
- In blood highest concentration is that of IgG.
- Fetal synthesis of IgM and IgA begin during the 5th month.
- Plasma cells can secrete IgM, IgG, IgA, IgE (all except IgD).
- IgA has 2 portions: J chain and S component.
- IgM also has J chain.
- Concentration in mg/ml = IgG: IgA: IgM: IgD: IgE = 12 : 1.8 : 1: 0-0.04: 0.00002
- Immunoglobulin present on the lymphocyte surface is IgD, and on basophils and mast cells is IgE.
- Half-lives:
IgG1, IgG2, IgG4- 23 days
IgG3- 7 days
IgA – 5.5 days
IgM- 5 days
IgD – 2.8 days
IgE – 2 days
- The placental passage is only for IgG.
- IgA is primarily present in body secretions.
- IgG and IgA are found in milk.
- Complement activation is mainly by IgM but also by IgG.
- IgM is the first type of antibody to be secreted during the primary response and they serve as receptors on the lymphocyte surface.
- Antiviral activity is highest with IgG and IgA (IgM also)
- Antibacterial activity is primarily by IgG, IgA, IgM.
- Antitoxin activity is only with IgG.
- Allergic activity is with IgE.
Conclusion
Humoral immunity is a very important component of the immune system. The production of antibodies is dependent on B-cell development and maturation. In the present discussion, we studied all the aspects of B-cell development and maturation. In a similar manner, T-cell development and maturation occur that makes the foundation for cell-mediated immune response. In the next articles, we shall read about the cell-mediated immune response.
References
References are available in the hardcopy of the website.

