Introduction to human immunodeficiency virus
The human immunodeficiency virus (HIV) is a retrovirus belonging to the family of lentiviruses which causes AIDS (acquired immune deficiency syndrome) in humans. Since the identification of this virus, extensive research has been done on HIV to understand its life cycle, routes of transmission, pathogenesis of AIDS and possible cure for the disease. Although attempts to develop anti-HIV drugs and vaccines have been ongoing for a long time, but still many aspects of HIV pathogenesis remain unclear. The main reason because of which the successful treatment of HIV infection is so difficult is “its rapid rate of evolutionary change”. We are still in a process to reconstruct the evolutionary history of HIV in order to develop better vaccines and antiviral agents. In 30% to 80% of HIV-infected individuals, oral manifestations of infection are observed. Oral lesions in AIDS are commonly associated with morbidity in HIV-infected cases. An in-depth understanding of AIDS and its oral manifestations is important for appropriate management of oral lesions in patients infected with HIV.
Historical aspect of HIV
AIDS was first identified in the USA in 1981. The HIV infection was found predominantly in promiscuous male homosexuals and intravenous drug abusers referred to as “AIDS risk groups” 1. This virus was first isolated in 1983 and soon it was evident that two types of HIV, with slightly different genome structures, were circulating in human populations 2. By the mid-1980’s, it became clear that the virus had spread, largely unnoticed, throughout most of the world. In 1986, WHO developed a provisional clinical AIDS case definition for adults and children 3. The number of cases and deaths among persons with AIDS increased rapidly during the 1980s, followed by substantial declines in new cases and deaths in the late 1990s. According to UNAIDS fact sheet 2014, in 2013 there were 35 million [33.2 million–37.2 million] people living with HIV. The report also highlighted that since the start of the epidemic, around 78 million [71 million-87 million] people have become infected with HIV and 39 million [35 million-43 million] people have died of AIDS-related illnesses 4.
The understanding of HIV started with the discovery of simian immunodeficiency viruses (SIVs) which were present in a wide variety of African primates 5. SIVs have been isolated from more than 20 African primate species, but it has been observed that SIVs do not cause disease in their hosts. Genetically and evolutionally there are two distinct types of human AIDS viruses: HIV-1 and HIV-2. Infection caused by HIV-1 is more severe as compared to HIV-2. Evidence suggests a chimpanzee subspecies (Pan troglodytes troglodytes) as the source of HIV-1 infection and the sooty mangabey (Cercocebus atys, a monkey found in West African rainforests between Senegal and Ghana) as the source of HIV-2 infection in human populations 6. HIV-1 is phylogenetically divided into three groups ‘M’, ‘N’ and ‘O’, with the M group further split into 9 subtypes and 15 circulating recombinant forms. The ‘M’ group of HIV-1 has ………… Contents available in the book…….. Contents available in the book…….. Contents available in the book…….. Contents available in the book……
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Structure of HIV
HIV consists of a cylindrical center surrounded by a sphere-shaped lipid bilayer envelope. The virus particle has a diameter of about 1/10,000 mm. Structurally, the virus is composed of three components: viral envelope, HIV matrix proteins, and the viral core.
Viral envelope:
As already stated, the viral envelope consists of a lipid bilayer. In the lipid bilayer, there are two major glycoproteins, gp120, and gp41. These glycoproteins are primarily involved in the recognition of CD4+ cells and chemokine receptors, thereby enabling the virus to attach to and invade CD4+ cells. HIV infects cells that carry the following receptor and co-receptor,
CD4: Expressed on the surface of CD4 T-lymphocytes (helper T-lymphocytes) and macrophages (including dendritic cells).
CCR5: Expressed on CD4+ T-lymphocytes and on macrophages.
CXCR4: Expressed on CD4+ T-lymphocytes and T-cell lines.
HIV matrix proteins:
The HIV matrix protein consists of p17 protein, which lies between the envelope and core.
Viral core:
It is formed of viral capsule protein p24 which surrounds two single strands of RNA and the enzymes needed for HIV replication, such as reverse transcriptase, protease, ribonuclease, and integrase. It also contains three out of nine virus genes, namely gag, pol, and env, that contain the information needed to make structural proteins for new virus particles.
‘gag’ gene contains around 1500 nucleotides. It gives rise to a 55-kilodalton (KD) gag precursor protein p55. This protein is later on cleaved into four smaller proteins which form the building blocks for the viral core,
- CA (Capsid protein p24)
- MA (Matrix protein p17) (this protein is a part of the “matrix” which anchors the core to the viral envelope)
- NC (Nucleocapsid protein p9)
- p6
‘pol’ gene encodes for enzymes required for various cellular processes in HIV which include,
- Protease
- RNAse H
- Integrase
‘env’ gene encodes for a single protein, gp160. After synthesis, this protein travels to the cell surface where it is split into two parts gp120 and gp41 by enzymatic action.
HIV life cycle
All viruses are obligate, intracellular parasites of cells, which mean that they require a living cell in order to reproduce. The life cycle of HIV starts in a host after its transmission from an infected person. The HIV is primarily transmitted by three routes: sexual, parenteral (blood-borne), and perinatal. Virtually all cases of HIV transmission can be attributed to these exposure categories. The sexual transmission of HIV can occur through male-to-female, female-to-male, male-to-male, and female-to-female sexual contact. The parenteral transmission may occur in recipients of infected blood or blood born products, intravenous or injection drug users who share needles, in health care providers through needlesticks and occasionally through mucous membrane exposure. Along with this, perinatal transmission from infected mother to child can occur in utero, during labor and delivery, or postpartum through breast-feeding. Cases of transmission of HIV infection from a patient to healthcare provider have been documented. Transmission occurs through parenteral or mucous membrane exposure to blood. However, the risk is less than 1% and can be further reduced by the availability of more effective Antiretroviral Therapy (ART) 7. The risk of HIV transmission through these routes is given in the following table,
| Risk of transmission of HIV through various modes | |
|---|---|
| Mode of transmission | Risk per exposure |
| Receptive anal and vaginal intercourse | ~ 0.1–3% and 0.1–0.2%, respectively, per episode |
| Insertive anal and vaginal intercourse | ~ 0.06% and 0.1%, respectively, per episode |
| Blood transfusion | ~ 95% per exposure |
| Sharing of needles by drug abusers | ~ 0.67% per exposure |
| Needlestick injuries | ~ 0.3–0.4% per exposure |
| Perinatal transmission | ~ 25–30% |
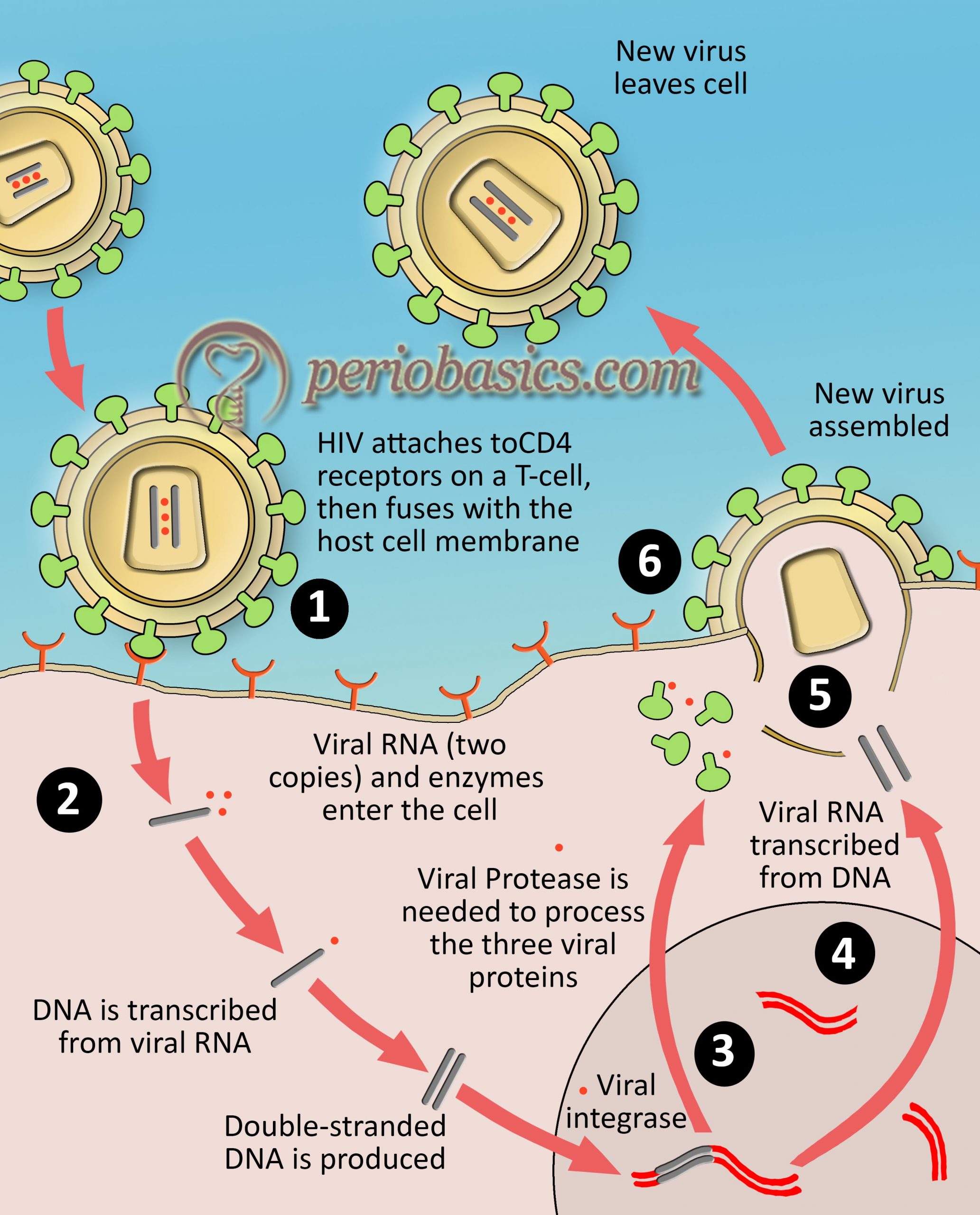
The life of HIV starts in a host with the fusion of gp120 glycoprotein on the HIV envelope with the CD4 receptor and one of two co-receptors, CCR5 or CXCR4, on the surface of a CD4+ T- lymphocyte. The virus then fuses to the host cell and releases its genetic material i.e. RNA into the host cell cytoplasm. Next step is the formation of viral DNA. Reverse transcriptase, an HIV enzyme converts the single-stranded HIV RNA to double-stranded HIV DNA. The newly formed HIV DNA then enters the host cell’s nucleus. In the nucleus, the viral DNA gets embedded into the host cell DNA with the help of a viral enzyme, DNA integrase. The integrated HIV DNA is called a provirus. This provirus may remain inactive for many years, producing few or no new copies of HIV. When the host cells get a signal of activation, the viral DNA starts producing copies of HIV genomic material by using the host cell enzyme, RNA polymerase. The m-RNA carrying information of viral proteins is synthesized and long chains of viral proteins are formed. The HIV proteases then cut the long protein chains into smaller individual proteins. These proteins assemble together to form viral structure to form a new viral particle. The newly formed virus particle “buds” out from the host cell. The virus possesses the surface glycoprotein receptors with which it attaches to the surface of new host cell.
Clinical signs and symptoms associated with HIV infection
After an individual gets infected with HIV, there is an initial burst of viral replication. The population of viral particles in the blood may reach 10,000,000/ml. The infected person may experience flu-like symptoms for up to 2 weeks. The patients usually complain of their condition as ‘worse flu ever’. Other symptoms include fever, swollen glands, sore throat, rash, muscle and joint aches and pains, fatigue, and headache. This phase of HIV infection is referred to as “acute retroviral syndrome” (ARS) or “primary HIV infection”. The seroconversion occurs after 3 to 8 week 14, 15. Because large numbers of viruses are produced during the initial period following infection, they use CD4 cells to replicate and destroy them in the process. Hence, there is a sharp downfall in CD4 cell count.
After the initial TH cell decline, the body starts generating an antibody response against HIV. This reduces the HIV count in the blood. The helper T-cell population recovers and the immune response is generated against the virus which keeps it at low and steady level. This level of virus in the blood is referred to as “viral set point”. Over a period of time, the immune system fails to cope up with the viral load due to infection of helper T-cells and viral levels rise again. The patient starts experiencing opportunistic infections such as Candida albicans infections of the mouth or vagina, persistent diarrhea, fever, weight loss and reactivation of previous infections such as shingles and tuberculosis. Eventually, the body fails to recover from these infections and the patient dies.
Case definition and staging of HIV
To diagnose a case of HIV infection, WHO has provided following case definitions,
Adults and children 18 months or older:
Positive HIV antibody testing (rapid or laboratory-based enzyme immunoassay). This is confirmed by a second HIV antibody test (rapid or laboratory-based enzyme immuno-assay) relying on different antigens or of different operating characteristics;
and/or
Positive virological test for HIV or its components (HIV-RNA / HIV-DNA/ ultrasensitive HIV p24 Ag) confirmed by second virological test obtained from a separate determination.
Children younger than 18 months:
positive virological test for HIV or its components (HIV-RNA / HIV-DNA / ultrasensitive HIV p24 antigen) confirmed by a second virological test obtained from a separate determination taken more than four weeks after birth. (Positive HIV antibody testing is not recommended for definitive or confirmatory diagnosis of HIV infection in children until 18 months of age).
The WHO (2005) clinical staging for HIV disease in adults/adolescents and children sorts the patients into one of the four hierarchical clinical stages, ranging from stage 1 (asymptomatic) to stage 4 (AIDS). Patients are assigned to a particular stage when they demonstrate at least one clinical condition in that stage’s criteria. Patients remain at a higher stage after they recover from the clinical condition which placed them in that stage.

Classification of oral lesions in HIV infection in adults
The first classification of oral lesions associated with HIV infection was given by the European Economic Community in 1986. The list contained 30 diseases which were commonly and less commonly associated with HIV infection. This classification was modified by Pindborg in 1989 17. In 1990, a set of definitions and diagnostic criteria for the common oral lesions seen in association with the HIV infection was proposed by a group of oral AIDS clinicians, epidemiologists, and pathologists 18. In august 1990, a revised classification was proposed based on the discussions during a 2-day EC-sponsored workshop held in Amsterdam 19. This revised classification proposed three groups of lesions, where Group I consisted of lesions strongly associated with HIV infection, Group 2 consisted of lesions less commonly associated with HIV infection and Group 3 was of those lesions only possibly associated with HIV infection. A fourth group was also defined which contained lesions associated with the use of drugs. Also, an attempt was made to clarify the clinical diagnostic criteria of the lesions and conditions listed in Group 1. This classification of the oral manifestations of HIV infections and their diagnostic criteria was again reviewed in 1992 20. Further, a group of authors defined 3 groups of oral manifestations of AIDS based on the intensity and clinical features 21.
Revised classification of oral lesions associated with HIV infection in adults
| GROUP I: Lesions strongly associated with HIV infection |
|---|
| A. Candidiasis · Erythematous · Pseudomembranous B. Hairy leukoplakia C. Kaposi's sarcoma D. Non-Hodgkin's lymphoma E. Periodontal disease · Linear gingival erythema · Necrotizing (ulcerative) gingivitis · Necrotizing (ulcerative) periodontitis |
| GROUP II: Lesions less commonly associated with HIV infection |
| A. Bacterial infections · Mycobacterium avium intracellulare · Mycobacterium tuberculosis B. Melanotic hyperpigmentation C. Necrotizing (ulcerative) stomatitis D. Salivary gland disease · Dry mouth due to decreased salivary flow rate · Unilateral or Bilateral swelling of major salivary glands E. Thrombocytopenic purpura F. Ulceration NOS (not otherwise specific) G. Viral infections · Herpes simplex virus · Human papillomavirus (warty-like lesions) · Condyloma acuminatum · Focal epithelial hyperplasia · Verruca vulgaris · Varicella-Zoster virus · Herpes zoster · Varicella |
| GROUP III: Lesions seen in HIV infection |
| A. Bacterial infection · Actinomyces israelii · Escherichia coli · Klebsiella pneumoniae B. Cat scratch disease C. Drug reactions (Ulceration, Erythema multiforme, Lichenoid reaction, Toxic epidermolysis) D. Fungal infections other than candidiasis · Cryptococcus neoformans · Geotrichum candidum · Histoplasma capsulatum · Mucoraceae (Mucormycosis/Zygomycosis) · Aspergillus flavus E. Neurologic disturbances · Facial palsy · Trigeminal neuralgia F. Recurrent aphthous stomatitis G. Viral infections · Cytomegalovirus · Molluscum contagiosum |
Classification of oral lesions associated with HIV infection based on intensity and features.
| GROUP I: Seven cardinal lesions that are strongly associated with HIV infection |
|---|
| Oral candidosis Hairy leukoplakia Kaposi sarcoma Linear gingival erythema Necrotizing ulcerative gingivitis Necrotizing ulcerative periodontitis Non-Hodgkin lymphoma |
| GROUP II |
| Atypical Ulcers Salivary glands diseases Viral infection such as cytomegalovírus (CMV), herpes simplex virus (HSV), papillomavirus (HPV), and herpes zoster virus (HZV). |
| GROUP II: Lesion rarer than those in groups 1 and 2 |
| Diffuse osteomyelitis Squamous cell carcinoma |
Classification of oral lesions in HIV-infected pediatric patients
According to World Health Organization (WHO), 2 million children had been infected with HIV worldwide by June, 1998. It represented 7% of the total estimated infected population of almost 30 million. In pediatric patients, the orofacial manifestations are among the earliest and commonest manifestations of HIV disease. Hence, oral health care providers play a key role in the identification of HIV infection in pediatric patients.
The guidelines for the diagnosis and management of HIV-related oral diseases in children were developed by the collaborative work group on the oral manifestations of pediatric HIV infection in March 1994 and May 1995. The framework was adapted from the classification system of the European Collaborative Clearing house on oral problems related to HIV infection and the WHO collaborating center on oral manifestations of the HIV 22.
| Orofacial lesions associated with pediatric HIV infection |
|---|
| GROUP I: Lesions commonly associated with pediatric HIV infection |
| A. Candidiasis · Pseudomembranous · Erythematous · Angular cheilitis B. Herpes simplex virus infection C. Linear gingival erythema D. Parotid enlargement E. Recurrent aphthous ulcers · Minor · Major · Herpetiform |
| GROUP II: Lesions less commonly associated with pediatric HIV infection |
| A. Bacterial infections of oral tissues B. Periodontal diseases · Necrotizing (ulcerative) gingivitis · Necrotizing (ulcerative) stomatitis · Necrotizing stomatitis C. Seborrheic dermatitis D. Viral infections · Cytomegalovirus · Human papillomavirus · Molluscum contagiosum · Varicella-Zoster virus i. Herpes zoster ii. Varicella E. Xerostomia |
| GROUP III: Lesions strongly associated with HIV infection but rare in children |
| A. Neoplasms · Kaposi's sarcoma · Non-Hodgkin's lymphoma B. Oral hairy leukoplakia C. Tuberculosis-related ulcers |
Anti-retroviral therapy
The anti-retroviral therapy (ART) has evolved tremendously since the introduction of zidovudine (AZT) which was approved by the US FDA in 1987 23. Zidovudine (AZT) is a nucleoside reverse transcriptase inhibitor (NRTI). Subsequently, more NRTI drugs were introduced but even in combination, they were not able to suppress the virus for long periods of time and patients still inevitably died 24. With extensive research on anti-retroviral drugs, highly active anti-retroviral therapy (HAART) was introduced. HAART involves a combination of multiple antiretroviral drugs. The treatment with HAART for HIV-infected patients was published by Hammer et al. (1997) 25 and Gulick et al. (1997) 26. The new therapy combining ………… Contents available in the book…….. Contents available in the book…….. Contents available in the book…….. Contents available in the book……
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Nucleoside analog reverse transcriptase inhibitors (NRTIs): e.g. Lamivudine, Didanosine, Abacavir, Emtricitabine, Zidovudine, Stavudine, and Tenofovir.
Non-nucleoside analog reverse transcriptase inhibitors (NNRTIs): e.g. Efavirenz, Delavirdine, Etravirine, and Nevirapine.
Protease inhibitors (PIs): e.g. Atazanavir, Darunavir, Saquinavir, Indinavir, Nelfinavir, and Ritonavir.
Integrase inhibitors (IIs): e.g. Dolutegravir and Raltegravir.
Fusion inhibitors (FIs): e.g. Enfuvirtide and Maraviroc.
Chemokine receptor antagonists (CCR5 antagonists): e.g. Aplaviroc, Vicriviroc, and Maraviroc.
Inspite of the introduction of HAART, which results in a marked rise in helper T-cells and a reduction in viral load to a point below the level of detection, the individual is still considered to be infected with HIV, because the virus remains sequestered somewhere in the body and becomes detectable in blood if medications are discontinued or if the virus becomes drug-resistant 27-30.
When to start ART?
Early detection and treatment of HIV has a great impact on improving survival and reducing the incidence of HIV infection at the community level. The HIV virus cannot be completely eradicated from the body with current medications, so the goals of ART include prolongation of life and improvement in the quality of life, greatest possible reduction in viral load for as long as possible, immune reconstitution that is both quantitative and qualitative, rational sequencing of drugs and reduction of HIV transmission by suppression of viral load. The WHO working group recommendations on when to start ART in adults, adolescents, pregnant and breastfeeding women and children are given in the following table,
| Summary of recommendations on when to start ART in adults, adolescents, pregnant and breastfeeding women and children | |
|---|---|
| Population | Recommendation |
| Infants < 1 year old | Initiate ART in all infants regardless of WHO clinical stage and CD4 cell count |
| Children 1–5 years Old | Initiate ART in all, regardless of WHO clinical stage and CD4 cell count |
| Children ≤5 years old | Initiate ART if CD4 cell count ≤500 cells/mm3 · Initiate ART in all children with severe/advanced HIV disease (WHO clinical stage 3 or 4) or CD4 counts ≤350 cells/mm3 Initiate ART regardless of CD4 cell count · WHO clinical stage 3 or 4 · Active TB disease |
| Adults and adolescents (≥10 years) | Initiate ART if CD4 cell count ≤500 cells/mm3 · As a priority, initiate ART in all individuals with severe/advanced HIV disease (WHO clinical stage 3 or 4) or CD4 counts ≤350 cells/mm3 Initiate ART regardless of WHO clinical stage and CD4 cell count in · Active TB disease · HBV coinfection with severe chronic liver disease · Pregnant and breastfeeding women with HIV · HIV-positive individual in a serodiscordant partnership (to reduce HIV transmission risk) |
Conclusion
HIV infection is presently a worldwide problem. There have been many efforts to make an effective medicine against this infection. We have managed to improve the quality of life of patients with HIV infection. However, we have still not been able to find an ideal medicine against HIV. There are many oral manifestations of HIV. In the next article, we shall read about these manifestations in detail. Read ” Oral manifestations of HIV and their management” for details.
References
References are available in the hard-copy of the website.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.