What is inflammation?
In simple words, inflammation is the response of the living tissue to injury. The word inflammation is derived from Latin word ‘inflammare’ (to set on fire). It is a well-organized sequence of events that take place in a living tissue following an injury due to mechanical trauma, chemical trauma, toxins and infection with pathogens which produce products that are harmful to the host. The credit for the first documentation of the four cardinal signs of inflammation goes to a Roman, Aulus Cornelius Celsus, more commonly known as Celsus (1st century AD). He was the first one to highlight first four signs of inflammation rubor et tumor cum calore et dolore (redness and swelling with heat and pain) 26, 27. The fifth sign of inflammation, function laesa (loss of function) was added by Virchow in 1871 28. With advances in microscopy, inflammation was defined at the cellular level. The historical milestones in our understanding of inflammation are given in the following table.
Researchers Observation and interpretation
Celsus (1st century AD) First documentation of the cardinal signs of inflammation.
Galen (3rd century AD) Described fifth sign of inflammation.
Virchow (1871) Introduced fifth sign of inflammation and stated that inflammation results in a pathological proliferation of cells due to leakage of nutrients from the vessels.
Cohnheim (1873) First described mechanism of diapedesis.
Metchnikoff (1908) Described inflammation as a defensive cellular response to pathogens which is primarily guided by vessels rather than pathology itself.
Lewis (1927) Described the vascular events during inflammation and the local chemical mediators involved in inflammation. First described neurogenic inflammation and physiological characterization of vascular events during inflammation.
Rocha e Silva (1974) Defined biochemical processes involved in inflammation.
Inflammatory response
The inflammatory response consists of various changes in the tissue, including circulatory (hemodynamic) changes, changes in the vessel wall permeability, response of white blood cells and release of soluble chemical mediators of inflammation. Let us discuss these changes in detail,
Circulatory (hemodynamic) changes
The vascular changes during the acute inflammatory reaction were described by Lewis in 1927 as ‘the triple response to injury’ following the application of a blunt instrument drawn firmly across the skin. These include flush (a dull red line follows due to capillary dilatation), flare (a red, irregular, surrounding zone then develops, due to arteriolar dilatation) and wheal (a zone of edema develops due to exudation into the extra-vascular space). A lot of research has been done since then.
The microcirculation of the soft tissue is made up of a network of small capillaries lying between arterioles, which have a thick muscular wall, and thin-walled venules. There is no smooth muscular layer in capillaries to control their diameter and these are quite narrow in diameter. Thus, the smooth muscle wall present in the arterioles controls the blood flow through the capillary bed by acting as a sphincter. Under normal conditions, the blood flow through the capillaries is ……..Contents available in the book……….Contents available in the book……….Contents available in the book……….Contents available in the book……
The vascular changes that occur during inflammation are as follows,
Increased vascular permeability
Blood vessels are mostly lined by a single layer of endothelial cells, which form a layer of uniform thickness around the vessel wall. These vessels, thus act as a micro filter, allowing the passage of water and solutes, but also act as a barrier for the passage of blood cells. Along with the transfer of fluid and solutes through ultra-filtration, there is diffusion of gasses like oxygen and carbon dioxide. The fluid that passes out of the vessels returns back to the vascular compartment because of high osmotic pressure inside the blood vessels due to the presence of plasma proteins. Thus, under normal conditions the fluid that is forced out of the blood vessels at the arteriolar end due to high hydrostatic pressure returns back into the blood vessels at the venous end due to low hydrostatic pressure. This balance between the hydrostatic and osmotic pressure in the blood vessels is essential for the maintenance of soft tissue health.
During acute inflammation, vasodilation at the arteriolar end is one of the earliest changes observed. It causes opening of new capillary beds in that area. This results in increased blood flow, causing heat and redness. Vasodilation is induced by the action of several mediators, notably histamine, and nitric oxide, on vascular smooth muscles (discussed later). Furthermore, the increased hydrostatic pressure at the arteriolar end results in the escape of more fluid along with plasma proteins into the extravascular compartment. The escape of plasma proteins into the extravascular compartment further facilitates fluid accumulation due to increase in osmotic pressure outside the vascular compartment. Thus, more fluid leaves the blood vessels than what is returned to them. This fluid, which contains plasma proteins is called as exudate.
Accumulation of cells and formation of cellular exudate
The accumulation of polymorphonuclear cells (PMN’s) in the extracellular space is the hallmark of acute inflammation. There is a specific manner in which PMN’s come out of the blood vessels and migrate towards the site of injury. The process of neutrophil migration from the blood vessels is termed as ‘transendothelial migration of neutrophils’. Now, let us discuss this procedure in detail.
Transendothelial migration of neutrophils
As already stated, under normal conditions, the blood cells are confined to the central stream in the blood vessel and not in the peripheral or plasmatic zone. However, following an injury, the acute inflammatory reaction is initiated which results in the loss of intravascular fluid and an increase in plasma viscosity with slowing of flow at the site of acute inflammation. The reduced flow rate of the blood allows neutrophils to flow in the plasmatic zone. Once, the neutrophils come near the wall of the blood vessel, the process of transendothelial migration is initiated. There are a number of receptor molecules that are involved in the process of leukocyte migration from inside the blood vessel to outside in the connective tissue. The knowledge of these receptors is essential to understand the mechanism of trans-endothelial migration.
Molecules involved in transendothelial migration:
The role of the majority of the following molecules in the trans-endothelial migration of leukocytes has been demonstrated by blocking the respective receptors by antibodies.
Selectins:
Selectins belong to a family of transmembrane molecules, expressed on the surface of leukocytes and activated endothelial cells. They are of 3 types; L-selectin, P-selectin, and E-selectin. The smallest of these is L-selectin, which is found on most leukocytes. P-selectin, the largest selectin, is expressed on activated platelets and endothelial cells primarily. E-selectin is expressed on activated endothelium during chemically or cytokine-induced inflammation. These contain an N-terminal extracellular domain with structural homology to calcium-dependent lectins, followed by a domain homologous to epidermal growth factor, and two to nine consensus repeats (CR) similar to sequences found in complement regulatory proteins. Each of these adhesion receptors is inserted ……..Contents available in the book……….Contents available in the book……….Contents available in the book……….Contents available in the book……
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Integrins:
These are transmembrane adhesive heterodimeric glycoproteins, made up of α and β subunits that function as receptors for the extracellular matrix. β subunit is known as CD18 and the α subunit is known as CD11. The α subunit is found in 4 forms, namely a, b, c, and d. So, based on α subunit variability, these glycoproteins are classified as,
CD11a/CD18, also known as lymphocyte function-associated antigen-1 (LFA-1),
CD11b/CD18, also known as macrophage receptor-1 (MAC-1),
CD11c/CD18 (p150, 95/CR4) and
CD11d/CD18 (αDβ2).
The principal receptors for ICAM-1 are the β integrins LFA-1 and MAC-1, and those for VCAM-1 are the integrins α4β1 and α4β7.
Intercellular adhesion molecules (ICAM-1 and ICAM-2):
These molecules perform many important tasks during trans-endothelial migration. They belong to the immunoglobulin superfamily and represent endothelial ligands for the leukocyte β-2 integrin, LFA-1. ICAM-1 is constitutively expressed on endothelial cells, platelets, and most leukocytes whereas, ICAM-2 appears to be concentrated at endothelial cell junctions.
Platelet endothelial cell adhesion molecule-1 (PECAM-1) or CD-31:
These molecules help in the endothelial cell to cell adhesion via homophilic interactions and they help in transendothelial migration through endothelium-leukocyte interactions. PECAM-1 homophilic interactions allow leukocytes to emigrate through the endothelial barrier 29. These belong to the immunoglobulin gene superfamily and are expressed on leukocytes, platelets, neutrophils, monocytes, and selected T-cell subsets 30.
Junctional adhesion molecules (JAMs):
JAMs are the members of the immunoglobulin gene superfamily. JAM proteins are localized in the intercellular junctions of polarized endothelial and epithelial cells. They are found in three forms JAM-A, JAM-B, and JAM-C. JAM-A is expressed at epithelial tight junctions and intercellular borders of endothelial cells, as well as on the surfaces of megakaryocytes. JAM-B and JAM-C are believed to be involved in leukocyte adhesion, transmigration, and interactions between different cell subsets during inflammation 31. Although JAM-A normally engages in homophilic adhesion, during inflammation it can bind to CD11a/CD18 on the leukocyte 32.
Vascular cell adhesion molecule-1 (VCAM-1):
It is an adhesion molecule that is not constitutively expressed on endothelial cells, but is upregulated by chemokines 33. This molecule has been shown to interact with monocytes and lymphocytes and participates in leukocyte transmigration during the inflammatory response 34.
VE-cadherin:
VE-cadherin is a transmembrane protein that establishes homotypic calcium-dependent interactions with its extracellular domain. It is one of the major components of adherence junctions. Although, many proteins have been implicated in endothelial cell-cell adhesion 34, 35; VE-cadherin has a central role in the regulation of the integrity of the endothelial barrier and leukocyte transmigration as evidenced in vitro and in vivo studies. The juxtamembrane domain binds p120 catenin, while the membrane distal domain binds β-catenin and plakoglobin in a mutually exclusive fashion that depends on cell-cell contact maturation. Finally, α-catenin alternately associates to β-catenin/plakoglobin or to the actin cytoskeleton.
CD99:
CD99 is a 32 kD, highly O-glycosylated molecule that is expressed on the surfaces of most leukocytes and is concentrated at the borders between confluent endothelial cells 35. Similar to PECAM-1, CD99 functions in a homophilic manner in the transmigration of leukocytes but CD99 regulates a later step in this process as compared to PECAM.
CD99L2 (CD99-like molecule 2):
It represents a protein of unknown function with moderate sequence homology to CD99, which is expressed on leukocytes and endothelial cells 36. Similar to PECAM, the homophilic interaction between CD99 at the endothelial cell border and CD99 on monocytes 37 and neutrophils 37 is required for transmigration.
Steps in transendothelial migration of leukocytes:
- Slow rolling,
- Adhesion strengthening,
- Intra-luminal crawling,
- Transendothelial migration,
- Migration through the basement membrane, and
- Interstitial migration
Step by step mechanism of transendothelial migration
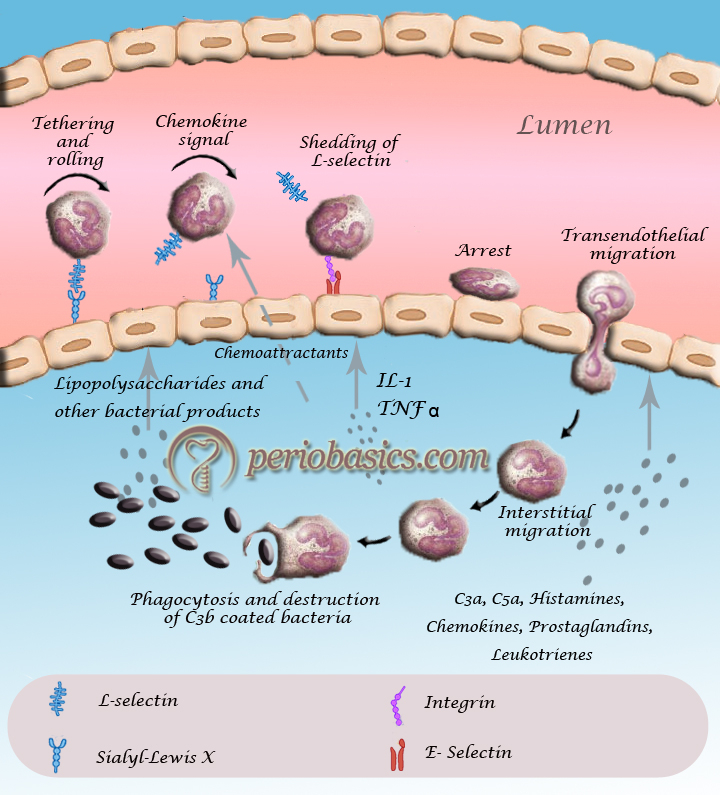
- As already stated, inflammation is a protective response of the body to any insult, which is manifested by the release of a variety of pro-inflammatory mediators from resident leukocytes and mast cells, including cytokines like IL-1β, TNF-α etc. Complement components like C3a and C5a are also important initiators of trans-endothelial migration of leukocytes.
- These mediators stimulate endothelial cells to express P-selectin and E-selectin on their luminal surfaces 37. Initial tethering and rolling are mediated by P-, E- and L-selectins 38. The initial rolling brings the leukocyte ……..Contents available in the book……….Contents available in the book……….Contents available in the book……….Contents available in the book…….
- After initial tethering and rolling the intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1) are expressed. Their binding to activated integrins probably contributes to adhesion stabilization and cell motility. In addition to mediating adhesion, integrins also generate intracellular signals that regulate various cellular functions 41, 42.
- Subsequently, leukocytes crawl inside the blood vessels in a MAC-1 and ICAM-1 dependent manner, seeking the preferred sites for diapedesis 43. After crawling, leukocytes migrate to a nearby endothelial border and squeeze between the tightly opposed endothelial cells to the underlying basement membrane in a next process of transendothelial migration (also called as diapedesis) 44. PECAM-1 (CD31) and CD99 act at sequential steps as the leukocyte crosses the endothelial barrier 45. Two main routes of leukocyte transendothelial migration are described in the literature:
i) The trans-cellular route, when leukocytes emigrate through the body of endothelial cells, and
ii) The para-cellular route, when leukocytes emigrate through junctions between adjacent endothelial cells 46, 47.
In trans-cellular migration of leukocyte ICAM-1 on endothelial cell engages with LFA-1 on leukocyte and induces the formation of micro-villi like endothelial cell projections embracing the migrating leukocyte in a cup-like structure 37, 48.
Most of the recently published studies identify, however, the para-cellular migration route as the main mechanism by which leukocytes emigrate from the intravascular compartment into the interstitium 47. Many cell contact proteins such as PECAM-1, members of the JAM family (JAM-A, JAM-B, and JAM-C), CD99, and ICAM-2 etc. are involved in the para-cellular migration of leukocyte into the extracellular matrix. - Ultimately, the leukocyte enters the extracellular matrix and under the chemoattractant gradient reaches the site of inflammation, where it performs its various functions.
Neutrophil-derived products and their functions
The activated neutrophils synthesize and secrete cytokines, chemokines, leukotrienes and prostaglandins, and by virtue of their accumulation in large numbers within the inflammatory tissue, they may contribute significantly to the local production of inflammatory mediators. The activated neutrophils have been shown to produce IL-1, IL-1RA, IL-6, IL-12, TGF-β and TNF-α 49. Furthermore, neutrophils also synthesize and secrete leukotrienes and prostaglandins, especially leukotriene B4 (LTB4) and prostaglandin E2 (PGE2), which are synthesized from arachidonic acid by lipoxygenases and cyclooxygenases pathways, respectively 50. Neutrophils also secrete matrix metalloproteinases (MMP-8 and MMP-9) which are involved in the degradation of connective tissue 51. These mediators along with causing inflammatory changes in the tissue, also activate other immune cells. A detailed description of neutrophils and their role in periodontal diseases has been given in “Role of neutrophils in host-microbial interactions”.
Chemical mediators of acute inflammation
The chemical mediators of inflammation can be broadly divided into systems based on their source and/or chemical composition. These are as follows,
Vasoactive amines:
Histamine (derived from mast cells and platelets).
Serotonin (derived from platelets).
Eicosanoids derived from arachidonic acid metabolism:
Prostaglandins.
Leukotrienes.
Cytokines:
Interleukin 1 (IL-1).
Interleukin 6 (IL-6).
Interleukin 11 (IL-11).
Tumor necrosis factor (TNF).
Products of phagocytosis:
Oxygen-derived free radicals.
Cationic proteins.
Neutral proteases.
Nitric oxide (NO)
Plasma protein systems:
Components of the complement system.
Kinin system.
Clotting/Fibrinolytic system.
The chemical mediators of inflammation can also be classified as preformed and newly formed mediators. The preformed mediators are already present in the cells, while the newly formed are synthesized during the acute inflammatory response. The primary preformed mediators include histamine (derived from mast cells, basophils, platelets), Serotonin (platelets) and lysosomes (neutrophils, macrophages) while newly formed mediators include prostaglandins (mast cells, leukocytes), leukotrienes (mast cells, leukocytes), platelet-activating factor (leukocytes, endothelial cells), reactive oxygen species (all leukocytes), nitric oxide (macrophages) and cytokines (lymphocytes and macrophages). Following is the brief description of these mediators,
Histamine:
It is a preformed mediator of inflammation and is the first one to be released during inflammation. The major sources of histamine include mast cells, basophils and eosinophil leukocytes, and platelets. The release of histamine is triggered by physical injury (trauma, heat, and cold), complement factors (C3a, C5a), neuropeptides (substance P), cytokines (IL-1, IL-8), an antigen-binding to mast cells and by lysosomal proteins released from neutrophils, including cationic proteins. Histamine is also ……..Contents available in the book……….Contents available in the book……….Contents available in the book……….Contents available in the book……
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Serotonin:
Serotonin (5-hydroxytryptamine) is a vasoactive amine, derived from the platelets. Its primary actions are vasodilation, increased vascular permeability, and platelet aggregation.
Prostaglandins:
These are the products of arachidonic acid metabolism. The four principal bioactive prostaglandins generated include prostaglandin E2 (PGE2), prostacyclin (PGI2), prostaglandin D2 (PGD2) and prostaglandin F2α (PGF2α). Out of these four prostaglandins, PGE2 is most abundant during acute inflammation, however, PGI2 also plays an important role in this process. PGD2 is primarily produced by mast cells. PGE2, PGI2, and PGD2 are powerful vasodilators individually and they also synergize with other inflammatory vasodilators like histamine and bradykinin. The vasodilatory effect of histamine and bradykinin is potentiated by PGE2, PGI2, and PGD2. Furthermore, these mediators do not produce pain themselves, but potentiate the afferent C fiber sensitization by histamine and bradykinin in causing pain.
Leukotrienes:
These mediators are synthesized from arachidonic acid by white blood cells through the action of soluble cytosolic enzymes. These are also synthesized by mast cells and platelets. 5-lipoxygenase oxidizes arachidonic acid to give an intermediary leukotriene (LTA4). Intracellular enzymes then convert LTA4 to either LTB4 or a series of cysteinyl-containing leukotrienes (LTC4, LTD4, and LTE4). Out of all the leuko-trienes, LTB4 acts as a potent chemotaxin and is present in inflammatory exudate. The cysteinyl-containing leukotrienes (LTC4, LTD4, and LTE4) cause an increase in vascular permeability.
Cytokines:
There are many cytokines involved in an acute inflammatory reaction, including IL-1, TNF-α, IL-6, IL-11, IL-8 and other chemokines, G-CSF, and GM-CSF (Read more in “Cytokines and their role in the pathogenesis of periodontal diseases”). Out of these, the most important cytokines involved in acute inflammation are IL-1 (α and β) and TNF. These cytokines mediate many of the effector functions of innate immunity and also act as the principal mediators of acute inflammatory response. IL-1 is secreted by neutrophils, endothelial cells, and epithelial cells while TNF is secreted by activated mononuclear phagocytes, natural killer (NK) cells and mast cells 53. Both IL-1 and TNF stimulate the release of chemokines from macrophages that enhances the affinity of leukocyte integrins for their ligands, thus facilitating trans-endothelial migration of leukocytes 54. IL-6 produced during acute inflammatory response acts both in innate and adaptive immunity 55. The majority of cells involved in inflammation, the cytokines produced by them and cytokines for which they have receptors are given in the following table,
Cells involved in inflammation, the cytokines produced by them and cytokines for which they have receptors
Cells Synthesize and secrete Respond to
Mast cells Histamine, IL-3, IL-4, IL-5, IL-6, GM-CSF, TNF-α IL-1, IL-3, IL-4, IL-9
Monocytes/ macrophages IL-1, IL-1Ra, TNF-α, IL-6, IL-8, IL-12, RANTES, MIG, IP-10, GM-CSF, G-CSF, TGF-β, IFN-α IL-1, TGF-β, GM-CSF, TNF-α, TNF-β, IFN-α
T-lymphocytes IL-1, IL-2, IL-2R, IL-3, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-13, GM-CSF, G-CSF, TGF-β, IFN-γ IL-1, IL-2, IL-4, IL-6, IL-7, IL-12, IL-15, IL-16, TGF-β
B-lymphocytes IL-1, IL-2, IL-3, IL-4, IL-5, IL-7, IL-14, IL-15, IFN-γ IL-4, IL-5, IL-13, TGF-β, INF-γ
Fibroblasts IL-1, IL-6, IL-11, GM-CSF, G-CSF, IFN-β IL-1, IL-4, IL-6, IL-17, TNF-α, TGF-β, IFN-γ
Neutrophils IL-1, IL-1, IL-6, IL-8, TNF-α, G-CSF, M-CSF IL-1, IL-2, IL-8, G-CSF, GM-CSF, TNF-α and β, MIP-1 and 2, TGF-β
Chemokines:
Chemokines are a large family of cytokines that play a highly important role in orchestrating the exquisitely organized and regulated movement of cells to specific locations within the body 56. Two initially discovered chemokines were IL-8 and monocyte chemoattractant protein (MCP)-1. The primary function of chemokines is to create a chemical gradient along which various immune cells (such as neutrophils, monocytes) move towards the site of injury. Furthermore, by attachment of chemokines to their receptors on neutrophils, eosinophils, basophils, mast cells and other cells, they trigger granule exocytosis, oxidative burst with the release of superoxide, and nitric oxide, and can affect gene expression, proliferation, homeostasis and apoptosis 57.
Reactive oxygen species:
The reactive oxygen species are a short-lived group of incredibly cytotoxic molecules released during inflammation. These significantly contribute to the tissue-damaging effects during inflammation 58-60. Although, certain levels of reactive oxygen species are required for normal metabolism, but during inflammation, they are produced in an excessive amount which causes tissue damage. The reactive oxygen species are primarily present in the form of superoxide, hydrogen peroxide, and lipid hydroperoxides. Neutrophils are the major source of free radicals at the site of inflammation.
Nitric oxide (NO):
This molecule has multiple modulating effects on inflammation and plays a key role in the regulation of immune responses. It is a short-lived molecule (half-life around 6 seconds), produced by enzymes known as nitric oxide synthases (NOSs). In tissues, nitric oxide (NO) is generated enzymatically by NOSs, which oxidize L-arginine to L-citrulline 61, 62. Because of its small size, this molecule can ……..Contents available in the book……….Contents available in the book……….Contents available in the book……….Contents available in the book……
Components of the complement system:
As already discussed, the complement system along with killing the invading microorganisms also orchestrates and connects various responses during immune and inflammatory reactions 63. The components of the complement system are involved virtually in all phases of inflammation, including changes in vascular flow and caliber, increase in vascular permeability, extravasation of leukocytes, and chemotaxis.
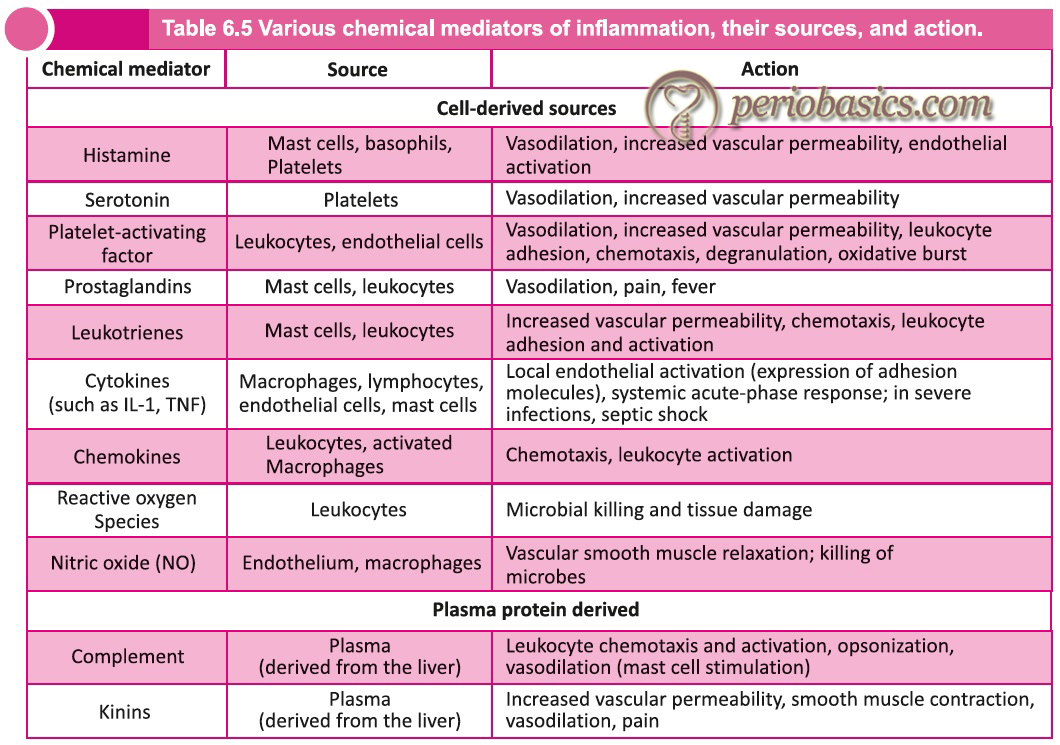
Kinins:
Kinins are important chemical mediators that produce many of the cardinal manifestations of inflammation. There are two major kinin families: the slow-acting bradykinins and the fast-acting tachykinins. The bradykinin family consists of bradykinin and Lys-bradykinin (also known as kallidin). These are formed by proteolytic cleavage of their protein precursor, kininogen, by plasma and tissue proteases known as kallikreins 64, 65. The cellular effects of these vasoactive kinin peptides are ……..Contents available in the book……….Contents available in the book……….Contents available in the book……….Contents available in the book……
Conclusion
The basic understanding of immunity and inflammation is essential to understand various aspects of periodontal disease progression. In the above discussion, we briefly discussed various aspects of innate as well as adaptive immunity. The interactions between microorganisms and host are complex and involve various chemical mediators. In the upcoming chapters, we shall read about the host-microbial interactions and various chemical mediators that are involved in the progression of periodontal diseases.
References
References are available in the hard-copy of the website.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.

