Introduction
Ribonucleic acid (RNA) is a crucial molecule involved in various biological roles, primarily in coding, decoding, regulation, and expression of genes. Unlike DNA, which is double-stranded, RNA is typically single-stranded and comes in several forms, each serving different functions within the cell. Types of RNA
Messenger RNA (mRNA): Carries genetic information from DNA to the ribosome, where proteins are synthesized.
Transfer RNA (tRNA): Helps decode mRNA sequences into proteins.
Ribosomal RNA (rRNA): Forms the core of ribosome’s structure and catalyzes protein synthesis.
Small nuclear RNA (snRNA): Involved in splicing of pre-mRNA.
MicroRNA (miRNA) and Small Interfering RNA (siRNA): Play roles in gene regulation through RNA interference.
RNA interference (RNAi) is a biological process that allows cells to regulate the expression of genes. Discovered in the late 1990s, RNAi has revolutionized our understanding of gene regulation and has become a powerful tool in molecular biology, with applications ranging from basic research to therapeutic development. In the following discussion, we shall explore the mechanisms of RNAi, its biological roles, technological applications, and potential in therapeutics.
Discovery and historical background
Historically, gene silencing phenomena was extensively explored by scientists in plants and other organisms. In the late 1980s and early 1990s, scientists studying plants noticed an unexpected phenomenon known as post-transcriptional gene silencing (PTGS). When they introduced additional copies of genes into plants to increase the expression of certain traits, instead of seeing increased gene activity, they observed a decrease in gene expression. This phenomenon, where both the introduced gene and the corresponding endogenous gene were silenced, was particularly evident in studies involving transgenic plants with added viral resistance genes. Similar observations were made around the same time in the study of Petunia. Researchers introduced extra pigment-producing genes, hoping to enhance flower color, but instead, the flowers exhibited less color than expected. This unexpected reduction in gene expression was called “co-suppression,” as both the introduced gene and the endogenous gene were suppressed. These observations hinted at a natural mechanism for gene silencing, but the underlying molecular basis remained unclear.
The breakthrough came in 1998 when Andrew Fire and Craig Mello published their seminal paper in the journal Nature, titled “Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans.” In their experiments with the nematode C. elegans, they demonstrated that the introduction of double-stranded RNA (dsRNA) corresponding to a specific gene could efficiently and specifically silence that gene’s expression. Fire and Mello discovered that this gene silencing was not due to the introduction of antisense RNA alone (as was previously thought) but was triggered by dsRNA. Their work revealed that dsRNA could degrade the messenger RNA (mRNA) of the corresponding gene, preventing its translation into protein. This process was both potent and specific, indicating a natural mechanism for regulating gene expression. The discovery of RNA interference was a monumental advancement in the field of genetics, earning Fire and Mello the Nobel Prize in Physiology or Medicine in 2006. Their work laid the foundation for understanding a fundamental biological process and opened up new avenues for genetic research and therapeutic applications.
Further studies revealed that the RNAi process involves several key components, including the Dicer enzyme and the RNA-induced silencing complex (RISC). Dicer processes the dsRNA into small interfering RNAs (siRNAs), which are then incorporated into the RISC. The RISC uses the siRNA as a guide to identify and degrade the corresponding mRNA, effectively silencing the target gene. Concurrently, the discovery of microRNAs (miRNAs) in the early 2000s further expanded the understanding of RNAi. miRNAs are endogenous, small non-coding RNAs that regulate gene expression by binding to target mRNAs and either degrading them or inhibiting their translation. The discovery of miRNAs highlighted the role of RNAi in regulating endogenous genes, beyond just the response to exogenous dsRNA.
Mechanisms of RNA interference
The mechanisms of RNA interference (RNAi) involve several well-coordinated steps that lead to the silencing of specific genes. Let us now discuss the process of RNA interference in detail,
Initiation: Introduction of Double-Stranded RNA (dsRNA)
RNA interference begins with the introduction of double-stranded RNA (dsRNA) into the cell. This dsRNA can come from various sources, such as viral infection, transposons, or artificially introduced RNA molecules. In some cases, dsRNA can be synthesized inside the cell from hairpin-shaped precursors or introduced exogenously in research and therapeutic settings. The dsRNA is recognized by the cellular machinery as a trigger for the RNAi pathway. This recognition is crucial for the cell to initiate the silencing process.
Processing of dsRNA by Dicer
Dicer is a ribonuclease enzyme that plays a central role in the RNAi mechanism. It belongs to the RNase III family of enzymes and is responsible for processing the dsRNA into smaller fragments. Dicer cleaves the long dsRNA into small RNA duplexes known as small interfering RNAs (siRNAs). These siRNAs are typically 20-25 nucleotides long. The siRNAs produced by Dicer consist of two strands: a guide strand (antisense strand) and a passenger strand (sense strand). These two strands are complementary to each other and form a duplex with overhanging ends.
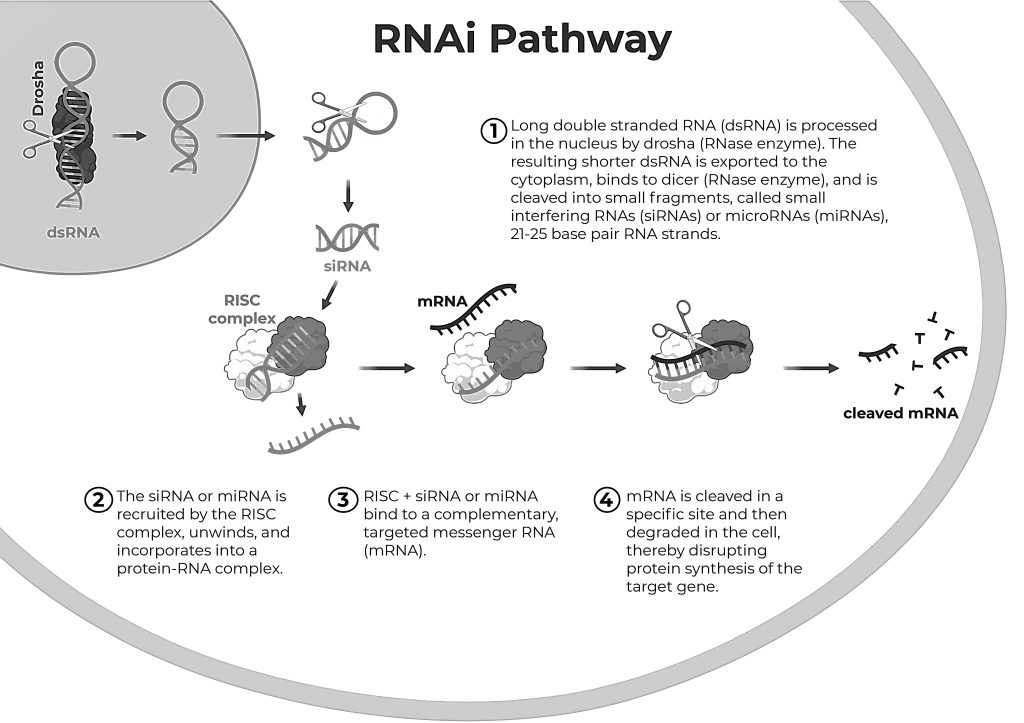
Assembly of the RNA-Induced Silencing Complex (RISC)
The siRNA duplex is then loaded into a multiprotein complex known as the RNA-induced silencing complex (RISC). RISC is a key component of the RNAi pathway and is responsible for mediating gene silencing. Within the RISC, the passenger strand of the siRNA is usually cleaved and discarded, while the guide strand remains associated with the complex. The selection of the guide strand is based on the thermodynamic stability of the two ends of the siRNA duplex. The strand with the less stable 5′ end is typically chosen as the guide strand. Once the guide strand is incorporated, RISC becomes an active complex capable of recognizing and binding to complementary target mRNAs.
Target recognition and binding
The guide strand within RISC guides the complex to its target mRNA by base-pairing with a complementary sequence within the mRNA. The degree of complementarity between the guide strand and the target mRNA determines the subsequent mode of action. There may be perfect or partial complementarity,
Perfect Complementarity (siRNAs): If the guide strand is fully complementary to the target mRNA (as in the case of siRNAs), the RNAi pathway typically leads to the cleavage and degradation of the mRNA.
Partial Complementarity (miRNAs): If the guide strand has partial complementarity to the target mRNA (as in the case of miRNAs), the RNAi pathway can lead to translational repression or destabilization of the mRNA without cleavage.
Gene Silencing
When the guide strand in RISC is fully complementary to the target mRNA, RISC, primarily through its Argonaute protein, cleaves the target mRNA at the site of base pairing. This cleavage typically occurs between the 10th and 11th nucleotides from the 5′ end of the guide strand. The cleaved mRNA is then rapidly degraded by cellular exonucleases, preventing it from being translated into protein. When the guide strand has partial complementarity to the target mRNA, RISC can inhibit translation of the mRNA without cleaving it. In some cases, this leads to the sequestration of the mRNA into processing bodies (P-bodies) in the cell, where it can be stored or eventually degraded.
Amplification and systemic spread (in some organisms)
In certain organisms, such as plants and nematodes (e.g., C. elegans), the RNAi response can be amplified. This amplification is mediated by RNA-dependent RNA polymerases (RdRPs). RdRPs synthesize new dsRNA from the cleaved mRNA fragments, which can be further processed by Dicer into additional siRNAs. This amplification mechanism enhances the silencing effect and ensures a more robust response. In some cases, the RNAi signal can spread from the initial site of dsRNA introduction to other cells and tissues in the organism. This phenomenon, known as systemic RNAi, allows for the silencing of genes throughout the organism. The exact mechanisms of systemic RNAi are still being studied, but it likely involves the transport of siRNAs or other RNA molecules between cells.
Termination and Recycling of RISC
After the target mRNA has been silenced, RISC can be recycled and used to target additional mRNA molecules that share the same sequence. This recycling process contributes to the efficiency of RNAi, as a single RISC complex can mediate the silencing of multiple mRNA molecules. The RNAi response eventually diminishes as the siRNAs or miRNAs are degraded or diluted through cell division. However, in some cases, persistent gene silencing can occur if the RNAi machinery continues to produce siRNAs from an ongoing source of dsRNA.
Biological Roles of RNA Interference
RNA interference serves several crucial biological functions, ranging from antiviral defense to the regulation of development and gene expression. Understanding these roles provides insight into the evolutionary significance of RNAi and its potential applications.
Antiviral defense
RNA interference (RNAi) plays a crucial role in the defense against viral infections, particularly in plants, invertebrates, and some vertebrates. This natural antiviral mechanism operates by targeting viral RNA for degradation, thus inhibiting viral replication and spread within the host organism. Many viruses, especially RNA viruses, produce double-stranded RNA (dsRNA) as part of their replication cycle. This dsRNA is a key trigger for the RNAi pathway. The dsRNA can be either the viral genome itself (in the case of some viruses) or replication intermediates that form during the synthesis of viral RNA. The host cell recognizes the viral dsRNA as foreign and activates the RNAi machinery to initiate an antiviral response. In plants and invertebrates, the presence of viral dsRNA is a primary signal for the induction of RNAi as part of the innate immune response. Upon recognition, the viral dsRNA is processed by the Dicer enzyme, a key component of the RNAi pathway. Dicer cleaves the long viral dsRNA into smaller fragments known as small interfering RNAs (siRNAs). These siRNAs are typically 21-24 nucleotides long and are crucial for the subsequent steps in the RNAi antiviral defense. The siRNAs generated by Dicer have two strands: a guide strand (antisense) and a passenger strand (sense). The guide strand is essential for targeting the viral RNA. The siRNA duplex is then incorporated into the RNA-induced silencing complex (RISC). Within RISC, the passenger strand is typically degraded, leaving the guide strand bound to the complex. The activated RISC, guided by the siRNA, is now capable of recognizing and binding to complementary sequences in the viral RNA. The guide strand within RISC base-pairs with complementary viral RNA sequences, allowing RISC to specifically target viral RNA molecules. Once the viral RNA is bound by RISC, the complex, particularly the Argonaute protein within RISC, cleaves the viral RNA at a specific site determined by the guide strand. This cleavage leads to the degradation of the viral RNA, preventing it from being translated into viral proteins and halting the replication process.
Regulation of gene expression
RNAi is a fundamental mechanism for regulating gene expression in eukaryotic cells. Through the precise targeting and silencing of specific mRNAs, RNAi plays a crucial role in various biological processes, including development, differentiation, and defense against pathogens. RNAi operates through two primary pathways involving small non-coding RNAs: small interfering RNAs (siRNAs) and microRNAs (miRNAs). Both of these pathways ultimately result in the downregulation of gene expression, but they do so in slightly different ways. siRNAs are typically derived from exogenous double-stranded RNA (dsRNA) or from endogenous long dsRNA molecules, such as those produced by transposons or viruses. The siRNA pathway primarily leads to the degradation of target mRNA, preventing its translation into protein. miRNAs are endogenous, single-stranded RNAs that are transcribed from the genome. They are processed from precursor miRNAs (pre-miRNAs) that form hairpin structures. The miRNA pathway usually results in translational repression or destabilization of the target mRNA, often without direct cleavage.
miRNAs are transcribed as primary miRNAs (pri-miRNAs) by RNA polymerase II. Pri-miRNAs are long transcripts that fold into hairpin structures. In the nucleus, the pri-miRNA is processed by the microprocessor complex, which includes the enzyme Drosha and the RNA-binding protein DGCR8, to produce precursor miRNAs (pre-miRNAs). The pre-miRNA is exported to the cytoplasm by Exportin-5, where it is further processed by the enzyme Dicer into a mature miRNA duplex. The mature miRNA duplex is loaded into the RNA-induced silencing complex (RISC), where one strand (the guide strand) is retained, and the other strand (the passenger strand) is typically degraded. The guide strand directs RISC to complementary sequences in target mRNAs. miRNAs often bind to the 3′ untranslated region (3′ UTR) of target mRNAs with imperfect complementarity, leading to translational repression. This prevents the ribosome from efficiently translating the mRNA into protein. In some cases, miRNA binding can lead to deadenylation and decapping of the mRNA, followed by degradation by exonucleases, thereby reducing the mRNA’s stability and preventing its translation.
Genome defense and transposon silencing
RNAi plays a crucial role in maintaining genome integrity by defending against transposable elements (transposons) and other genomic invaders. Transposons are DNA sequences that can move or “transpose” themselves to different locations within the genome, potentially causing mutations, disrupting gene function, or leading to genome instability. RNAi-mediated transposon silencing is an essential mechanism for controlling these elements and ensuring the stability of the genome. There are two types of transposons: DNA Transposons- These elements move through a “cut-and-paste” mechanism, where they are excised from one location in the genome and inserted into another; and Retrotransposons- These move via a “copy-and-paste” mechanism, where the transposon is transcribed into RNA, which is then reverse-transcribed into DNA and inserted into a new genomic location. Transposons can cause harmful mutations if they insert into or near essential genes, disrupt regulatory elements, or cause chromosomal rearrangements. To prevent such potentially deleterious effects, cells have evolved mechanisms to suppress transposon activity, with RNAi being a key player.
Transposons, especially retrotransposons, are transcribed into RNA as part of their lifecycle. These RNAs can be recognized by the host cell as potentially harmful. The RNAi machinery detects these transposon-derived RNAs and targets them for silencing. Transposon RNAs can form double-stranded RNA (dsRNA) either through their inherent secondary structure (e.g., hairpins) or through the activity of RNA-dependent RNA polymerases (RdRPs) that convert single-stranded transposon RNAs into dsRNA. These dsRNAs serve as the primary trigger for the RNAi pathway. The mechanism of silencing is the same as described in the previous sections.
Epigenetic regulation
Epigenetic regulation by RNA interference (RNAi) is a crucial mechanism that controls gene expression without altering the underlying DNA sequence. This regulation occurs through modifications to chromatin structure, DNA methylation, and the establishment of transcriptionally repressive environments. RNAi plays a significant role in establishing and maintaining these epigenetic marks, particularly through the processes of heterochromatin formation and transcriptional gene silencing. The epigenetic mechanisms involve the following,
DNA Methylation: The addition of a methyl group to the 5-position of cytosine residues in DNA, often leading to gene silencing.
Histone Modifications: Chemical modifications to histone proteins, such as methylation, acetylation, phosphorylation, and ubiquitination, which affect chromatin structure and gene expression.
Chromatin Remodeling: The reorganization of chromatin from a loosely packed (euchromatin) to a tightly packed (heterochromatin) state, influencing gene accessibility.
Non-Coding RNAs: Small RNAs, including those involved in RNAi, play a role in guiding epigenetic modifications to specific genomic regions.
Heterochromatin is a condensed form of chromatin that is transcriptionally inactive. It is enriched with specific histone modifications, such as trimethylation of histone H3 at lysine 9 (H3K9me3), and is associated with gene silencing. Small interfering RNAs (siRNAs) are generated from double-stranded RNA (dsRNA) precursors by the enzyme Dicer. These siRNAs are then incorporated into the RNA-induced silencing complex (RISC). siRNAs guide RISC to complementary RNA transcripts, typically derived from repetitive elements or transposons. These RNA transcripts are often produced in regions of the genome that are destined to become heterochromatin. Once RISC is bound to its target, it recruits chromatin-modifying enzymes, such as histone methyltransferases (e.g., SUV39H1), which add repressive histone marks like H3K9me3 to the surrounding chromatin. This mark is recognized by chromatin-binding proteins, such as HP1 (heterochromatin protein 1), which help condense the chromatin into a heterochromatic state. RNAi not only silences gene expression post-transcriptionally but also plays a direct role in transcriptional gene silencing by modifying the chromatin state of gene promoters or other regulatory regions. In some organisms, siRNAs can guide DNA methylation machinery to specific genomic regions. For example, in plants, siRNAs direct the de novo DNA methyltransferases, such as DRM2, to target sequences, leading to cytosine methylation at CpG, CHG, and CHH sites (where H = A, T, or C). siRNAs can also guide histone-modifying enzymes to target loci. The deposition of repressive histone marks, such as H3K9me3, is a key event in the establishment of transcriptionally inactive chromatin. This prevents the binding of transcription factors and RNA polymerase, effectively silencing gene transcription. Polycomb group (PcG) proteins are another class of epigenetic regulators that contribute to TGS. RNAi pathways can interact with PcG proteins to help establish and maintain repressive chromatin domains, particularly at developmental genes that must remain silenced in certain cell types.
RNAi and DNA methylation pathways often work together to reinforce gene silencing. For instance, in plants, siRNAs guide both histone modification and DNA methylation machinery to target loci, creating a robust silencing environment. RNAi-induced histone modifications can lead to chromatin remodeling, further enhancing the silencing of specific genes or genomic regions. The interaction between RNAi and chromatin remodelers, such as SWI/SNF complexes, helps maintain a repressive chromatin structure. RNAi contributes to the establishment of epigenetic memory, where silencing marks are maintained through cell divisions. This ensures that once a gene or genomic region is silenced, it remains inactive in daughter cells. The repressive marks established by RNAi, such as H3K9me3 and DNA methylation, can be recognized and propagated by reader proteins during DNA replication, ensuring the inheritance of the silenced state.
Technological Applications of RNA Interference
The discovery of RNA interference has led to the development of powerful tools for studying gene function and manipulating gene expression in various organisms. These applications have had a profound impact on basic research, biotechnology, and medicine.
Gene knockdown in model organisms
Gene knockdown in model organisms using RNA interference (RNAi) is a powerful technique for studying gene function. By selectively reducing the expression of specific genes, researchers can observe the resulting phenotypes, thereby gaining insights into the role of those genes in various biological processes. RNAi-mediated gene knockdown has been successfully applied in a wide range of model organisms, including Caenorhabditis elegans (C. elegans), Drosophila melanogaster (fruit fly), mice, plants, and others. Let us first try to understand the difference between gene knockdown and gene knockout. In gene knockdown, RNAi reduces gene expression but does not completely eliminate it, often resulting in a partial loss of function, which can be useful for studying essential genes. On the other hand, in gene knockout involves the complete inactivation of a gene, often through genetic engineering techniques like CRISPR/Cas9.
elegans was the first organism in which RNAi was discovered and characterized, making it a pivotal model for studying gene knockdown. The discovery was awarded the Nobel Prize in Physiology or Medicine in 2006 (discussed earlier). Drosophila is a well-established model for studying development, genetics, and neurobiology. RNAi has become an essential tool for functional genomics in this organism. RNAi in Drosophila has been used to study a wide range of biological processes, including development, immune responses, and behavior. It is also used in large-scale genetic screens to identify gene functions and interactions.
High-throughput screening
High-throughput screening (HTS) using RNA interference (RNAi) is a powerful approach for systematically studying gene function on a large scale. By employing RNAi to silence or reduce the expression of thousands of genes across a genome, researchers can identify genes that play critical roles in various biological processes, diseases, and cellular pathways. This technique has been instrumental in functional genomics, drug discovery, and understanding complex biological systems. HTS leverages automation, miniaturization, and large-scale data analysis to test the effects of RNAi on a vast number of genes simultaneously. RNAi, as a tool for gene silencing, fits perfectly into HTS methodologies, allowing for the systematic investigation of gene function. RNAi can be delivered via small interfering RNAs (siRNAs), short hairpin RNAs (shRNAs), or other RNAi-based constructs, which are introduced into cells or organisms to knock down target gene expression.
Functional genomics
Functional genomics and RNA interference (RNAi) are closely intertwined fields that collectively advance our understanding of gene function and regulation. Functional genomics focuses on the systematic analysis of gene functions and interactions within the context of the whole genome, while RNAi serves as a powerful tool to achieve gene silencing, enabling researchers to study the impact of reduced gene expression on cellular and organismal phenotypes. Functional genomics aims to determine the roles of genes and their products (proteins or RNAs) in various biological processes. It seeks to understand how genes contribute to phenotypes, cellular functions, and complex traits. As already discussed above, gene knockdown can be done by silencing specific genes with the help of which researchers can study the resulting phenotypic changes and infer gene function. This helps identify genes involved in essential processes such as cell cycle regulation, apoptosis, and signal transduction. Furthermore, RNAi screens can reveal the roles of genes within specific signaling pathways, providing insights into how pathways are regulated and how they interact with each other. RNAi is used to create cellular or animal models of diseases by knocking down genes associated with particular conditions. This approach helps in understanding disease mechanisms and identifying potential therapeutic targets. In cancer research, RNAi screens identify genes that, when knocked down, selectively kill cancer cells with specific genetic mutations. This strategy helps in discovering potential drug targets and developing targeted therapies. RNAi screens help identify and validate new drug targets by revealing genes whose knockdown affects cell viability, proliferation, or other relevant phenotypes. RNAi can also be used to identify genes that influence the sensitivity or resistance of cells to drugs, aiding in the development of combination therapies and personalized medicine.
RNAi can provide functional information about genes with unknown functions, aiding in the annotation of genomes and the assignment of functions to previously uncharacterized genes. By analyzing the effects of simultaneous knockdown of multiple genes, RNAi screens can reveal genetic interactions and regulatory networks. RNAi can sometimes affect non-target genes due to partial sequence complementarity. Researchers use bioinformatics tools and experimental validation to minimize and account for off-target effects. Confirmation of results using independent RNAi constructs or alternative methods (e.g., CRISPR) helps ensure that observed phenotypes are due to specific gene knockdown.
Synthetic biology
Synthetic biology involves designing and constructing new biological parts, devices, and systems, as well as re-engineering existing biological systems for useful purposes. It combines principles from engineering, biology, and computer science to create novel functions and organisms. As already discussed, RNA interference provides a method to regulate gene expression by targeting and degrading specific mRNA transcripts. This allows for precise control over gene activity, which is crucial for constructing and managing synthetic biological systems. RNAi can be used to modulate the activity of genes within synthetic circuits, enabling dynamic control over gene expression and allowing for fine-tuning of circuit behavior. In synthetic biology applications, RNAi can be employed to deactivate synthetic genes or pathways when they are not needed, reducing the risk of unintended effects or biosafety concerns. RNAi can be used to regulate the expression of key enzymes or components in synthetic metabolic pathways, optimizing production of desired products.
RNAi allows researchers to modulate the expression of genes within synthetic genetic circuits, enabling fine-tuning of circuit outputs. This can be used to create circuits with specific responses to environmental inputs or cellular conditions. RNAi can be integrated into synthetic circuits to enhance signal processing and feedback mechanisms, improving the reliability and performance of engineered biological systems. Along with this, RNAi can regulate key enzymes in synthetic metabolic pathways, optimizing the production of valuable compounds, such as pharmaceuticals, biofuels, or industrial chemicals. So, it can be used to implement feedback control mechanisms in synthetic metabolic pathways, maintaining optimal levels of intermediates and final products.
RNA Interference in therapeutics
RNA interference (RNAi) has emerged as a promising tool in therapeutics, offering the potential to precisely target and modulate gene expression for the treatment of a wide range of diseases. By harnessing the natural RNAi mechanism, researchers are developing innovative therapies that can address genetic disorders, cancer, viral infections, and other conditions. In case of genetic disorders RNAi can be used to treat various diseases. In monogenic diseases, RNAi can be used to silence mutant or overexpressed genes responsible for genetic disorders. For example, therapies targeting specific mutations in genes associated with cystic fibrosis or muscular dystrophy are being developed. For diseases caused by dominant negative mutations (where the mutant gene interferes with the function of the normal gene), RNAi can specifically target and degrade the mutant transcript. In cancer therapy, RNAi can target and inhibit the expression of oncogenes (genes that promote cancer growth) or cancer-driving mutations. For instance, RNAi-based therapies are being explored to target genes like MYC or KRAS that are frequently mutated in cancers. RNAi can also be used to downregulate genes that inhibit the expression or function of tumor suppressors, thereby promoting cancer cell death or sensitizing cancer cells to other treatments.
In viral infections, RNAi can target viral RNA and inhibit replication. This approach has been used to develop therapies for viral infections such as hepatitis B and C, as well as HIV. RNAi-based strategies are being explored to develop broad-spectrum antiviral treatments by targeting conserved viral elements.
In neurodegenerative diseases, RNAi can target and reduce the expression of pathogenic proteins involved, such as in Alzheimer’s or Parkinson’s disease. For example, RNAi is being investigated to target mutant forms of the huntingtin gene in Huntington’s disease. Delivery methods are being optimized to achieve effective RNAi-based therapy in the brain, including using viral vectors or nanoparticles that can cross the blood-brain barrier. In cardiovascular disorders, RNAi can be used to target genes involved in cholesterol metabolism or inflammatory pathways to treat atherosclerosis or other cardiovascular diseases. Along with this, RNAi-based therapies are being developed to target genes associated with heart failure or myocardial infarction.
RNAi therapeutics for genetic disorders
RNA interference (RNAi) therapeutics hold significant promise for the treatment of genetic disorders by targeting and modulating the expression of specific genes involved in these conditions. RNAi-based therapies work by silencing or downregulating the expression of mutant or overactive genes, offering a potentially transformative approach for managing a range of genetic diseases. Cystic fibrosis is caused by mutations in the CFTR gene, leading to defective chloride channels and thick mucus production. RNAi therapeutics aim to target and silence the mutant CFTR mRNA, potentially reducing the production of the defective protein and alleviating symptoms. In muscular dystrophy various forms of muscular dystrophy are caused by mutations in genes such as dystrophin (DMD) or lamin A/C (LMNA). RNAi can be used to reduce the expression of the mutant proteins or to correct splicing defects, potentially improving muscle function and stability. Huntington’s disease is caused by a mutation in the HTT gene, leading to the production of a toxic polyglutamine-expanded huntingtin protein. RNAi therapeutics aim to target and degrade the mutant HTT mRNA, reducing the production of the toxic protein and slowing disease progression. In diseases caused by dominant negative mutations, the mutant protein interferes with the function of the normal protein, such as in certain types of collagenopathies. RNAi can be used to specifically target and degrade the mutant mRNA, thereby reducing the production of the dominant negative protein and alleviating its effects. Some genetic disorders are caused by gain-of-function mutations where the mutated gene product has abnormal or toxic activity. RNAi can be used to silence the overactive gene, reducing the production of the aberrant protein and mitigating its harmful effects.
Antiviral RNAi therapies
Given RNAi’s natural role in antiviral defense, it is not surprising that RNAi-based therapies have been explored as a treatment for viral infections. RNAi can be used to target and degrade viral RNAs, thereby preventing the production of viral proteins and inhibiting viral replication. This approach has been investigated for a variety of viral infections, including hepatitis B and C, human immunodeficiency virus (HIV), and influenza. For example, in the case of hepatitis B, RNAi-based therapies have been designed to target the viral mRNAs encoding key viral proteins, such as the surface antigen (HBsAg) and the viral polymerase. By reducing the levels of these proteins, RNAi can suppress viral replication and potentially lead to the clearance of the virus from infected cells.
RNAi in Cancer Therapy
RNA interference holds significant promise for the treatment of cancer, as it can be used to selectively target oncogenes, genes that drive the growth and survival of cancer cells. RNAi-based therapies have been developed to silence the expression of oncogenes such as KRAS, MYC, and BCL2, which are commonly overexpressed in various types of cancer. In addition to targeting oncogenes, RNAi can be used to inhibit the expression of genes involved in drug resistance, angiogenesis, and metastasis. For instance, RNAi-based therapies targeting the expression of VEGF (vascular endothelial growth factor) have been explored as a means to inhibit tumor angiogenesis, the process by which tumors develop new blood vessels to sustain their growth.
Challenges and Strategies for RNAi Delivery
Effective delivery of RNA interference (RNAi) therapeutics is one of the major challenges in translating RNAi technology into clinical practice. The success of RNAi-based therapies depends significantly on the ability to deliver RNA molecules efficiently to the target cells or tissues while minimizing off-target effects and immune responses. Following are the challenges and strategies for RNAi delivery,
Challenges for RNAi Delivery
Targeted Delivery
Achieving targeted delivery to specific tissues or cell types is challenging. RNAi therapeutics need to be delivered specifically to the cells where they will be most effective, such as those affected by a particular disease. RNAi molecules must cross cellular membranes and barriers, such as the blood-brain barrier or extracellular matrix, to reach their targets.
Stability and Degradation
RNAi molecules are prone to degradation by nucleases in the bloodstream or extracellular environment. Ensuring the stability of RNAi therapeutics is crucial for effective delivery and function. The pharmacokinetics of RNAi molecules, including their distribution, metabolism, and excretion, need to be optimized to achieve therapeutic levels at the target site.
Immune Response
RNAi molecules can trigger immune responses, leading to inflammation or reduced therapeutic efficacy. Minimizing immune activation is essential for the safety and effectiveness of RNAi-based therapies. RNAi molecules can sometimes silence non-target genes with similar sequences, leading to unintended effects. Ensuring specificity is important to avoid off-target silencing.
Delivery Efficiency
Efficient cellular uptake of RNAi molecules is a significant challenge. RNAi molecules need to enter cells in sufficient quantities to achieve effective gene silencing. Once inside cells, RNAi molecules must escape endosomes to reach the cytoplasm, where they can exert their effects. Overcoming endosomal entrapment is crucial for effective delivery.
Strategies for RNAi Delivery
Nanoparticle-Based Delivery:
Lipid nanoparticles (LNPs) are widely used for RNAi delivery due to their ability to encapsulate RNA molecules and protect them from degradation. LNPs can also facilitate cellular uptake and endosomal escape. Polymers such as polyethylenimine (PEI) or poly(lactic-co-glycolic acid) (PLGA) can be used to form nanoparticles that carry RNAi molecules. These nanoparticles can be engineered for improved stability and targeted delivery.
Viral Vectors:
Adenoviral vectors can deliver RNAi constructs to a wide range of cell types. They are effective for transient expression but can elicit immune responses. Lentiviral vectors also provide stable integration of RNAi constructs into the host genome, allowing for long-term gene silencing. They are useful for applications requiring sustained RNAi effects.
Chemical Modifications:
Modifying RNAi molecules with chemical modifications (e.g., 2′-O-methylation, phosphorothioate linkages) can enhance their stability, reduce immune activation, and improve efficacy. RNAi molecules can be conjugated to targeting ligands (e.g., antibodies, peptides) to enhance tissue or cell specificity.
Targeted Delivery Systems:
Nanoparticles can be functionalized with ligands that specifically bind to receptors on target cells, improving the selectivity of RNAi delivery. Aptamers (short oligonucleotides) can be used to target specific cell types or tissues, enhancing the delivery of RNAi therapeutics.
Physical Methods:
Electroporation involves applying an electric field to cells to create transient pores in the cell membrane, allowing RNAi molecules to enter the cells. This method is used for both in vitro and in vivo applications. Microparticle bombardment (also known as gene gun technology) uses high-velocity microparticles coated with RNAi molecules to deliver them into cells or tissues.
Encapsulation and Delivery Vehicles:
Hydrogels can encapsulate RNAi molecules and provide controlled release, improving the stability and sustained delivery of RNAi therapeutics. Natural extracellular vesicles, such as exosomes, can also be used as delivery vehicles for RNAi molecules, benefiting from their biocompatibility and ability to cross biological barriers.
Future Directions and Ethical Considerations
As RNA interference continues to advance as a research tool and therapeutic strategy, several future directions and ethical considerations must be taken into account.
CRISPR and RNAi Synergy
The advent of CRISPR-Cas9 gene-editing technology has provided new opportunities for combining RNAi with genome editing. By integrating RNAi with CRISPR-based approaches, researchers can achieve more precise and efficient gene modulation. For example, RNAi can be used to transiently knock down gene expression before permanent gene editing with CRISPR, allowing for a more controlled and reversible approach to gene therapy.
Personalized Medicine
RNAi-based therapies hold great promise for personalized medicine, where treatments are tailored to an individual’s genetic makeup. Advances in genomic sequencing and biomarker discovery will enable the identification of specific gene targets for RNAi-based therapies, allowing for more personalized and effective treatments for a wide range of diseases.
Ethical Considerations
As with any emerging technology, RNAi-based therapies raise ethical considerations that must be carefully addressed. These include issues related to off-target effects, unintended gene silencing, and the long-term consequences of gene modulation. Ensuring the safety and efficacy of RNAi-based therapies through rigorous preclinical and clinical testing is essential to minimize risks and maximize benefits for patients.
Additionally, the accessibility and affordability of RNAi-based therapies are important ethical considerations. Ensuring that these therapies are available to all patients, regardless of socioeconomic status, will be critical to their equitable distribution and impact on public health.
Conclusion
RNA interference has revolutionized our understanding of gene regulation and has emerged as a powerful tool in molecular biology and medicine. Its ability to selectively silence specific genes has provided invaluable insights into gene function and has opened up new avenues for therapeutic development. From basic research to personalized medicine, RNAi has the potential to transform the way we study and treat diseases.
References
References are available in the hardcopy of the website “Periobasics: A Textbook of Periodontics and Implantology”.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users: