Introduction
Hyaluronic acid (HA) is a naturally occurring substance in the body, found primarily in connective tissues, skin, and eyes. It is a glycosaminoglycan, a type of long, unbranched polysaccharide composed of repeating disaccharide units. Unlike other glycosaminoglycans, HA does not contain sulfur and is not sulfated. It forms a coiled linear chain in a well-hydrated configuration. In mammals, its molecular weight can vary widely, ranging from 20 to 4,000 kilodaltons (kDa). Structurally, it is composed of,
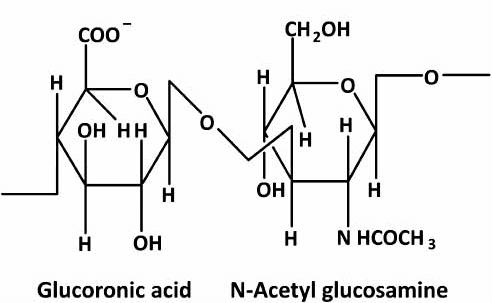
Repeating Disaccharides: The basic repeating unit of HA is composed of D-glucuronic acid and N-acetyl-D-glucosamine. These disaccharides are linked together by alternating β-(1→3) and β-(1→4) glycosidic bonds.
High Molecular Weight: HA can have a molecular weight ranging from 103 to 107 Daltons, which contributes to its unique viscoelastic properties.
HA is synthesized by hyaluronan synthases (HAS1, HAS2, and HAS3) located in the plasma membrane. These enzymes polymerize the disaccharide units by alternating the addition of glucuronic acid and N-acetylglucosamine from their respective UDP-sugar precursors. HA interacts with specific binding proteins called hyaladherins (e.g., CD44, RHAMM) that mediate its biological functions. Through its interactions with cell surface receptors, HA can influence various signaling pathways involved in inflammation, angiogenesis, and immune responses. HA is degraded by enzymes called hyaluronidases, which cleave the glycosidic bonds between the disaccharide units. The turnover rate of HA is rapid in tissues such as the skin and synovial fluid, with half-lives ranging from hours to days.
Biological functions of hyaluronic acid
Hydration:
HA’s ability to attract and retain water molecules (up to 1,000 times its weight) is crucial for maintaining tissue hydration and turgidity.
Extracellular Matrix:
HA is a major component of the extracellular matrix, providing structural support and facilitating cell migration and proliferation.
Wound Healing:
It plays a role in tissue repair and regeneration by promoting cell migration and proliferation.
Lubrication:
It’s a major component of synovial fluid, enhancing joint lubrication.
Cartilage Resilience:
In articular cartilage, HA surrounds chondrocytes (cartilage cells) and contributes to cartilage’s resistance to compression.
Cell Proliferation and Migration:
As part of the extracellular matrix, HA influences cell behavior.
Medical applications of hyaluronic acid
Hyaluronic acid (HA) has a range of medical applications due to its biocompatibility, ability to retain moisture, and role in tissue regeneration and repair. HA is available in the form of face creams, serums, and eye drops. Many people use it to promote skin health and combat signs of aging. HA-based dermal fillers are commonly used for facial rejuvenation. They help reduce wrinkles, restore volume, and enhance skin elasticity. HA aids in tissue regeneration and wound healing by maintaining moisture and regulating the repair process. HA injections are directly injected into joints (such as the knee) to lubricate them. This approach is particularly helpful for people with arthritis experiencing joint pain and inflammation. HA is used during cataract surgery, corneal transplantation, and other eye surgeries to maintain eye shape, protect delicate tissues, and facilitate the manipulation of ocular tissues. HA is used in coatings for cardiovascular implants and devices to improve biocompatibility and reduce the risk of thrombosis. The above stated are only few applications of HA in medical fields. There are many other uses of HA in various medical fields which are discussed elsewhere.
Periodontal applications of hyaluronic acid
The use of HA in periodontal therapy has gained attention due to its anti-inflammatory, anti-bacterial, and wound-healing properties. Several studies have highlighted the efficacy of HA in managing gingivitis and periodontitis. A study by Xu et al. (2004) demonstrated that topical application of HA significantly reduced gingival inflammation and bleeding in patients with chronic periodontitis. The anti-inflammatory effects were attributed to HA’s ability to modulate cytokine levels, reducing pro-inflammatory mediators such as IL-1β and TNF-α. The wound-healing properties of HA are well-documented. A randomized controlled trial by Smejkalová et al. (2009) found that HA gel applied post-surgically in patients undergoing periodontal flap surgery enhanced soft tissue healing and reduced postoperative pain. The study suggested that HA promotes fibroblast proliferation and collagen synthesis, which are crucial for tissue repair.
The topical application of HA as an adjunct to periodontal treatment has also been investigated. Sahin et al. (2017) evaluated the effectiveness of HA in the treatment of gingivitis. It was found that topical application of HA gel significantly reduced gingival inflammation and bleeding on probing compared to a placebo. Lauritano et al. (2015) in a clinical trial involving patients with chronic periodontitis reported that the adjunctive use of HA gel after scaling and root planing resulted in greater reductions in periodontal pocket depth and clinical attachment loss compared to scaling and root planing alone. HA-based mouthwashes and gels have been shown to be effective adjuncts in periodontal therapy. A meta-analysis by Pistorius et al. (2005) reviewed several clinical trials and concluded that HA mouthwashes significantly improved clinical parameters, such as plaque index and bleeding on probing, compared to placebo. The analysis emphasized the importance of HA concentration and molecular weight in determining clinical outcomes. Research by Ballini et al. (2015) demonstrated that HA application after scaling and root planing (SRP) resulted in greater reduction of periodontal pocket depth and improvement in clinical attachment levels compared to SRP alone. The study hypothesized that HA’s viscoelastic properties help stabilize the periodontal tissue and facilitate healing. The application of HA in cases of peri-implantitis has also been found to be effective. Fabbri et al. (2012) reported that HA gel applied around dental implants with peri-implantitis reduced inflammation and bacterial load, leading to improved peri-implant health. The study highlighted HA’s potential as an adjunctive treatment in implantology (References available in book).
Jentsch et al. (2014) assessed the effects of HA on tissue regeneration in periodontal defects. The results indicated that HA application, combined with bone graft materials, significantly improved the clinical outcomes in terms of new bone formation and attachment gain. In the context of gingival recession, HA has been used as a regenerative agent. A study by Kozlovsky et al. (2007) showed that the application of HA in conjunction with coronally advanced flap (CAF) procedures led to significant root coverage and improved gingival thickness, compared to CAF alone. The regenerative effects were linked to HA’s ability to enhance angiogenesis and cell migration.
Commercial products with HA in periodontal use
Several commercial products leverage HA for periodontal applications. These products aim to enhance healing, reduce inflammation, and improve clinical outcomes. Some of these include,
HA-Based Gels: These gels are applied locally to periodontal pockets during nonsurgical or surgical therapy. They promote tissue repair and reduce postoperative inflammation.
HA-Containing Dermal Fillers: While primarily used for cosmetic purposes, some dermal fillers containing HA have been explored for periodontal tissue regeneration.
HA-Infused Barrier Membranes: These membranes are used in guided tissue regeneration procedures to enhance wound healing and prevent epithelial migration.
HA-Coated Sutures: HA-coated sutures may improve tissue integration and reduce inflammation at suture sites.
Future applications of Hyaluronic acid in periodontal therapy
Hyaluronic acid (HA) has shown great promise in various areas of medicine and dentistry, including periodontal therapy. Here are some potential future applications of hyaluronic acid in this field:
Enhanced Tissue Regeneration:
HA’s role in wound healing and tissue regeneration could be further explored to improve periodontal tissue regeneration. Future applications might include HA-based scaffolds or gels that promote the repair of periodontal tissues, potentially enhancing the outcomes of regenerative procedures.
Targeted Delivery Systems:
HA could be used to develop advanced drug delivery systems that release therapeutic agents in a controlled manner. This would allow for targeted delivery of medications directly to the periodontal tissues, increasing their effectiveness and reducing systemic side effects.
Bioactive Coatings for Implants:
Incorporating HA into the surface coatings of dental implants might improve osseointegration and reduce inflammation. HA could promote better integration of the implant with surrounding bone and soft tissue, leading to improved long-term success rates.
Treatment of Periodontal Pockets:
HA-based gels or pastes could be used to fill and treat periodontal pockets. These formulations might not only aid in the mechanical removal of pathogens but also provide a bioactive environment that supports healing and reduces pocket depth.
Adjunctive Therapies:
HA might be used as an adjunct to conventional periodontal treatments. For instance, HA could be combined with other therapeutic agents like antimicrobial peptides or growth factors to enhance the overall efficacy of periodontal therapy.
Personalized Medicine:
Advances in genomics and proteomics could allow for the development of HA formulations tailored to individual patient needs. Personalized HA therapies could be designed based on specific periodontal conditions or genetic factors influencing healing and disease progression.
Improved Diagnostic Tools:
Future developments might include HA-based diagnostic tools that can help in the early detection of periodontal disease. For example, HA could be used in conjunction with imaging technologies or biomarkers to identify disease onset and monitor treatment progress.
Nanotechnology:
HA could be engineered into nanoparticles or nanofibers for controlled and sustained release of therapeutic agents, allowing for more precise targeting of periodontal tissues.
Growth Factors and Biomolecules:
Combining HA with growth factors (e.g., Platelet-Derived Growth Factor) or other biomolecules could enhance tissue regeneration and healing more effectively.
Gene Therapy:
Exploring HA in conjunction with gene therapy techniques to deliver genetic material that promotes tissue repair and regeneration.
These applications could significantly advance the management of periodontal diseases, making treatments more effective, less invasive, and tailored to individual patient needs.
Conclusion
Hyaluronic acid (HA) therapy in periodontal treatment leverages the natural properties of HA to enhance the healing and regeneration of oral tissues. A personalized HA treatments tailored to the specific needs of an individual based on their genetic profile or specific periodontal condition could improve outcomes. As research advances, HA therapy is likely to become increasingly sophisticated, offering more targeted, effective, and personalized treatment options for periodontal disease and tissue regeneration.
References
References are available in the hardcopy of the website “Periobasics: A Textbook of Periodontics and Implantology”.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:

