Introduction to the healthy periodontium
Periodontium refers to a group of specialized tissues that surround and support the teeth, maintaining them in the maxillary and mandibular bones. The word ‘Periodontium’ is derived from the Greek words peri-, meaning “around” and -odons, meaning “tooth”. Periodontium comprises of root cementum, periodontal ligament, bone lining the tooth socket (alveolar bone), and the part of gingiva facing the tooth surfaces (dentogingival junction). All of these tooth-supporting structures are different in terms of tissue architecture, biochemical and chemical composition. They perform distinct functions and are capable of adapting to the changes in the environment around them. The unique functions that these tissues carry out are,
Attachment: They attach the teeth to their bony housing and also to one another.
Resistance: These tissues resist and resolve the forces produced during mastication, speech, and deglutition.
Adaptation: These tissues have the capability to adapt to the changes in the external environment and wear associated with aging through a continuous process of remodeling and regeneration.
Defense: They have an internal defense mechanism that protects them against the noxious stimuli present in the oral cavity.
Periodontology is the scientific study of the periodontium in health and in disease 1. Periodontics is that specialty of dentistry which encompasses the prevention, diagnosis, and treatment of diseases of the supporting and surrounding tissues of the teeth or their substitutes; the maintenance of the health, function, and esthetics of these structures and tissues; and the replacement of lost teeth and supporting structures by grafting or implantation of natural and synthetic devices and materials 1.
A healthy periodontium is required to adequately support the teeth in function. The structural integrity and interactions between the tooth-supporting structures are the fundamental requirements for a healthy periodontium. Various disease processes around the teeth result in the destruction of periodontal tissues, thus making them mobile. If the destructive process continues, the tooth/teeth are ultimately lost. The first step in our journey to understand Periodontology starts with a thorough understanding of the normal periodontium and structure of periodontal tissues in health. In the following discussion, we shall discuss in detail various tooth-supporting structures, their development, structural organization, function and their ability to adapt to the changes in the surrounding environment.
Development of orofacial structures
After fertilization of the egg, there occurs a precisely coordinated cascade of developmental processes involving cell migration, growth, differentiation and apoptosis which results in the development of craniofacial structures. During the third week of development, the cranial end of the embryo undergoes precocious development where an oropharyngeal membrane (bucco-pharyngeal , or oral membrane) is formed at the site of the future face, between the primordium of the heart and the rapidly enlarging primordium of the brain. It is composed of the ectoderm externally and the endoderm internally. During the fourth week of development, this membrane breaks down in order to form the opening between the future oral cavity (primitive mouth or stomodeum) and the foregut.
During the fourth week of development, the cranial region of the human embryo resembles a fish embryo at a comparable stage. The human face begins to form during the 4th week of embryonic development and by the 6th week, the external face is completed. The formation of the external face takes place from two sources: the tissues of the frontonasal process that cover the forebrain (predominantly of neural crest origin); and the tissues of the first (mandibular) pharyngeal arch (mixed mesoderm and neural crest origin). The frontonasal process gives rise to a pair of medial nasal processes (that later contribute to a single globular [intermaxillary] process), and a pair of lateral nasal processes. The mandibular arch gives rise to a pair of mandibular processes (actually the pharyngeal arch itself), and a pair of the outgrowths of the arch- the maxillary processes (that later give rise to a pair of palatal processes).

Development of teeth and periodontium
By the fifth week of development, a horseshoe-shaped band of thickened epithelium (dental lamina) forms on the developing maxillary and mandibular bones. These are essentially the primordial dental arches. Neural crest cells, which are derived from the neural tube play an important role in the development of teeth. These cells are of ectodermal origin migrating into mesenchymal tissue; therefore are also referred to as ectomesenchymal cells. The dental lamina is comprised of cells that proliferate at a faster rate as compared to the adjacent epithelial cells. Later on during development, at predetermined sites on the dental lamina corresponding to ten deciduous teeth, further cellular proliferation takes place forming small pro-tuberances. These protuberances give rise to deciduous teeth. The development of teeth takes place in three distinct phases based on the characteristics of the developing teeth, the bud, bell and cap stages. In the “bud stage”, also referred to as primordia of enamel organ, two types …………………..Content available in the hard-copy of the website…………………………….Content available in the hard-copy of the website………..
Periobasics A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
The “bell stage” is characterized by the formation of two principal hard tissues of the tooth, enamel, and dentin (Figure 1.2a, 1.2b). Enamel is formed by ameloblasts derived from the terminal differentiation of cells from the inner epithelium of enamel organ and dentin is formed by odontoblasts derived from mesenchymal cells of the dental papilla. The dental follicle gives rise to cementoblasts, osteoblasts, and fibroblasts which are responsible for the formation of the tooth-supporting structures. In the later stages of the bell stage (also referred to as advanced bell stage), the growth of cervical loop cells into the deeper tissues forms Hertwig’s epithelial root sheath, which determines the root shape of the tooth. The cementoblasts derived from the dental follicle deposit cementum on the root surface and fibroblasts give rise to the periodontal ligament. As the root formation continues, the osteoblasts deposit bone around the root of the tooth. Eventually, mature tooth structure is formed which is supported by the supporting tissues, i.e. cementum, periodontal ligament, and bone; invested in the gingiva. Now, let us discuss in detail the components of the periodontium.
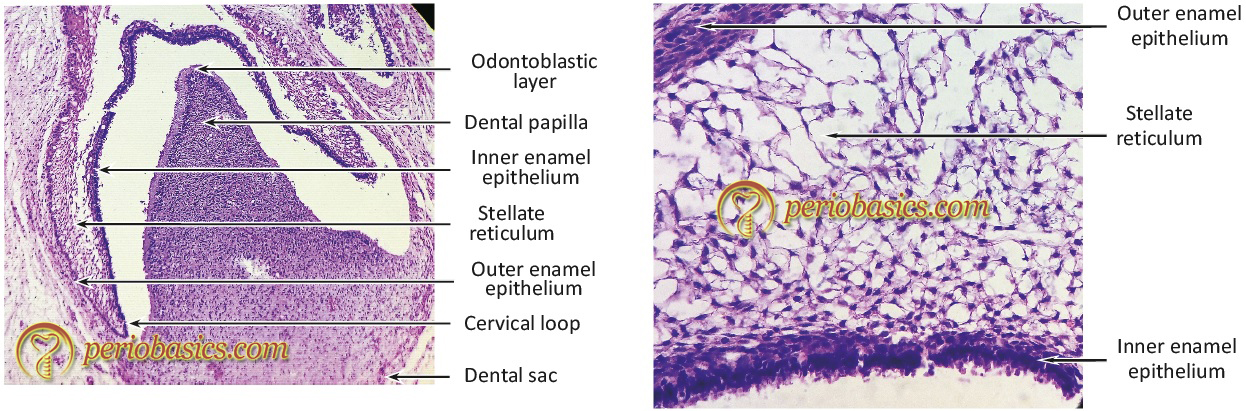
Gingiva
The oral mucosa has been traditionally divided into three categories: lining mucosa, specialized mucosa, and masticatory mucosa. The lining mucosa constitutes about 60% of the total oral mucosa. It is relatively loosely bound to the adjacent structures by the connective tissue that is rich in elastin. It covers the floor of the mouth, ventral (underside) tongue, alveolar mucosa, cheeks, lips, and soft palate. It does not function during mastication and therefore is non-keratinized, soft and pliable. Specialized mucosa makes around 15% of the total oral mucosa. It covers the dorsal surface of the tongue and composed of cornified epithelial papillae. Masticatory mucosa is the load-bearing mucosa during mastication. It is usually keratinized. It constitutes around 25% of the total oral mucosa and is present as gingiva (free, attached and interdental) and covers the hard palate.
Gingiva is that portion of the oral mucosa which covers the tooth-bearing part of the alveolar bone and the cervical neck of the teeth. It is typically coral pink in color, but its color may vary due to physiologic pigmentation among some races. It exhibits no exudate in periodontal health.
Macroscopic features of gingiva
The gingiva can be anatomically divided into marginal, attached and interdental gingiva.
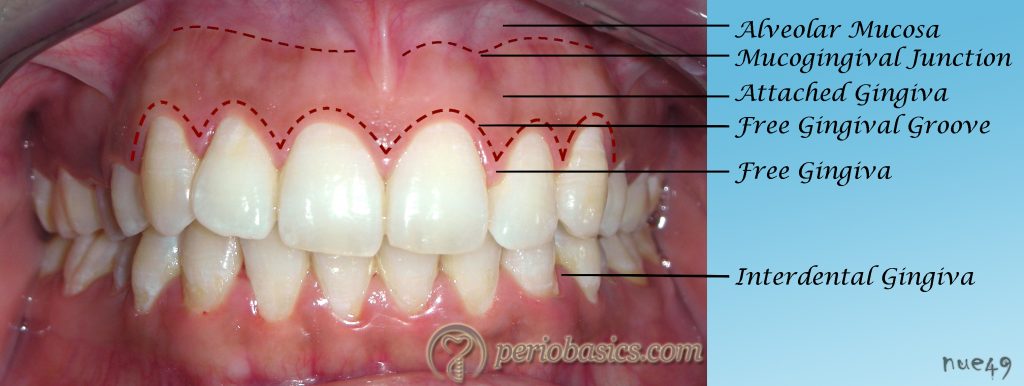
Marginal gingiva:
The marginal gingiva or unattached gingiva forms the coronal border of the gingiva which surrounds the tooth but is not adherent to it. In normal periodontal tissues, it extends approximately 2 mm coronal to the cementoenamel junction (CEJ). It is demarcated from the attached gingiva by a shallow linear depression, the free gingival groove in approximately 50% of cases 3. Histologically, the marginal gingiva is made up of oral gingival epithelium coronal to the gingival groove, oral sulcular epithelium, junctional epithelium and subjacent connective tissue of the lamina propria. In the absence of inflammation and pocket formation, the gingival groove runs somewhat parallel to and about 0.5 to 1.5 mm from the gingival margin 4, and it is approximately at the level of the bottom of the gingival sulcus.
Gingival sulcus:
A shallow space between the marginal gingiva and the external tooth surface is termed as gingival sulcus. The boundaries of the gingival sulcus are,
Inner: Tooth surface which may be the enamel, cementum, or a part of each, depending on the position of the junctional epithelium.
Outer: Sulcular epithelium.
Base: Coronal margin of the attached tissues.
The normal depth of the gingival sulcus and the corresponding width of the marginal gingiva is variable. Under absolutely ideal conditions, the sulcus depth is 0 or close to 0 mm 5. This condition can only be achieved in germfree animals or after prolonged and stringent plaque control 6, 7. The histological studies have reported the sulcus depth of 1.8 mm in healthy periodontium with a variation of 0-6 mm 8. Others have reported sulcus depth of 1.12 to 2.91 mm 9 and 0.69 mm 10. In general, sulcular depth less than 2 to 3 mm in humans and animals is considered as normal 11. The depth of gingival sulcus is an important indicator of periodontal status. The inflammation in the periodontal tissues due to plaque accumulation results in the conversion of normal sulcus into a pathological pocket. However, it must be remembered that the depth of a sulcus histologically (histological sulcus depth) is not necessarily the same as the depth which could be measured with a periodontal probe (clinical sulcus depth). Histological sulcus depth is considered as the exact sulcus depth. The sulcus depth determined by probing may be more than the histological depth if the periodontal probe penetrates the connective tissue, especially when it is inflamed or it may be less when the periodontal probe does not reach the bottom of the sulcus.

Attached gingiva
The attached gingiva is continuous with the oral epithelium of the free gingiva and is firmly bound to the underlying periosteum of the alveolar bone. It extends from the base of the free gingiva to the mucogingival junction (Figure 1.5) where the keratinized epithelium of attached gingiva abruptly merges with the non-keratinized epithelium of the alveolar mucosa 3. The mucogingival junction is a stable landmark which is probably genetically determined 12. The attached gingiva is usually ‘stippled’, with small regularly spaced depressions on its surface, giving it an “orange peel appearance” 13, 14. It is considered as a sign of healthy gingiva but it must be remembered that the presence or absence of stippling alone cannot determine the gingival health 15.
In different areas of the mouth, the width of attached gingiva varies. It is usually greatest in the incisor region (3.5 to 4.5 mm in the maxilla and 3.3 to 3.9 mm in the mandible). In the posterior areas, it is less with the least width in the first premolar area (1.9 mm in the maxilla and 1.8 mm in the mandible) 3. A variation of 1-9 mm in the width of attached gingiva has been reported in humans 16.
In 1972, Lang and Löe16 in a study reported that ………………….Content available in the hard-copy of the website……………………………. Content available in the hard-copy of the website………..
Periobasics A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
The mean width of attached gingiva increases from the primary dentition to permanent dentition 23. The anatomical width of attached gingiva increases slightly with the increasing age because of tooth eruption to compensate for occlusal wear 12.
Interdental gingiva
The interdental gingiva occupies the interproximal spaces between the adjoining teeth. The shape of the interdental gingiva is determined by the contact areas of the adjoining teeth and their mesiobuccal, mesiolingual, distobuccal and distolingual line angles. In the anterior teeth, the interdental gingiva assumes the conical shape and is referred to as interdental papilla. Generally, the papillary surface is keratinized. In the posterior teeth, the apex of the interdental gingiva is blunted with buccal and lingual peaks. This shape is referred to as “Col”. Col is a depression between the buccal and lingual papillae which conforms to the interproximal contact area 24. The dimensions of the col are determined by the width of the contact area between adjoining teeth. Because it represents the area of fusion of junctional epithelium of two adjoining teeth, it is non-keratinized and is more susceptible to damage from plaque and other noxious stimuli as compared to the keratinized gingiva.
Know More…
Periodontal biotype (phenotype):
In 1969, Ochsenbein and Ross 25 described two types of gingival forms: flat and highly scalloped. They observed that flat gingival anatomy was found in patients having square teeth while the highly scalloped gingival form was found in patients with a tapered tooth form. Seibert and Lindhe (1989) 26 later used the term periodontal biotype to describe gingival forms and classified gingiva as thin scalloped or thick-flat. The term periodontal phenotype is used inter-changeably with the term periodontal biotype. The term biotype has been replaced by the term phenotype in the recent (2017) world workshop classification system. Periodontal biotype refers to the hereditary thickness of periodontal tissue. The normal/thin periodontal biotype is found in around 75% of the patients, whereas thick biotype is found in approximately 25%. A thick periodontal biotype displays a thick and wide gingiva, wider teeth and thicker bone. These patients are less likely to have gingival recession, but more likely to have exostoses and intrabony defects during periodontitis 27. Many methods 28-45 have been used to determine the gingival thickness including injection needles, transgingival probing, histologic sections, cephalometric radiographs, probe transparency, ultrasonic devices, CBCT and conventional histology on cadaver jaws.
Microscopic features of gingiva
Microscopically, the gingiva can be studied under three headings,
- Gingival epithelium
- Epithelium-connective tissue interface, and
- Gingival connective tissue.
Gingival epithelium
The gingival epithelium can be further divided into three functional compartments: outer gingival epithelium, sulcular epithelium, and junctional epithelium.
Outer gingival epithelium
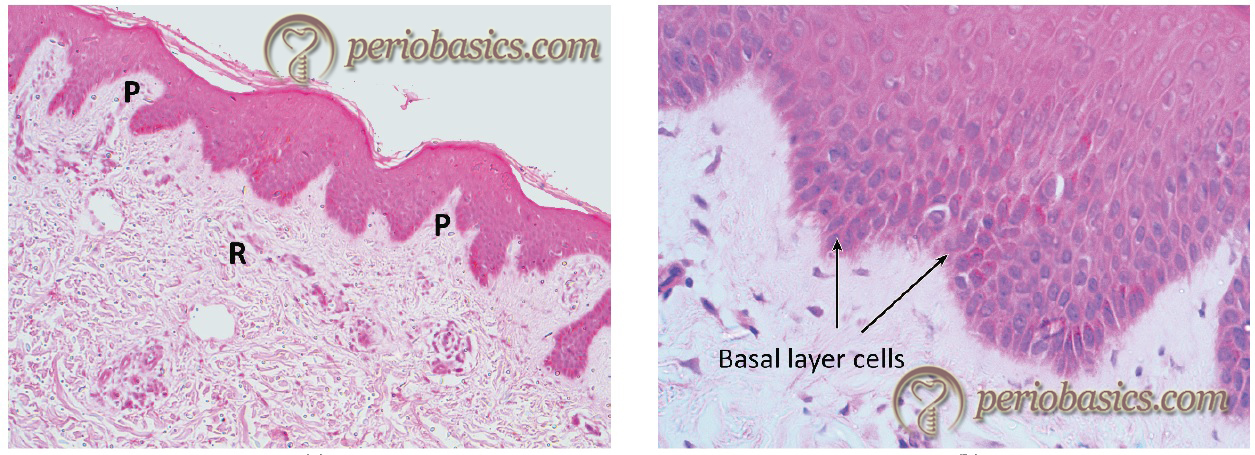
The outer gingival epithelium consists of keratinized stratified squamous epithelium, which covers the attached gingiva and the crest and outer surface of the marginal gingiva. The principal cells of the gingival epithelium are the keratinocytes. The non-keratinocytes associated with the gingival epithelium are melanocytes, Langerhans cells, and Merkel cells 46-48. The epithelium is organized into four layers which are distinguishable microscopically. These layers are basal cell layer (stratum basale), spinous cell layer (stratum spinosum), granular cell layer (stratum granulosum), and the cornified /keratinized cell layer (stratum corneum).
The basal layer makes the proliferation compartment of the epithelium, whereas the remaining layers make the differentiation compartment. The gingival epithelium is firmly attached to the underlying connective tissue and is nonpermeable to water-soluble substances. The proliferation of the keratinocytes takes place by mitosis primarily in the basal layer and to some extent in the suprabasal layers. The basal layer consists of one or two layers of cuboidal cells, which are undifferentiated cells. These cells then migrate to the suprabasal layers and differentiate to form mature keratinocytes. A small number of cells remain in the proliferative compartment of the basal layer, participating in the formation of new cells. These are attached to the lamina lucida zone of the basement membrane with hemidesmosomes. The lamina densa zone of the basement membrane faces the connective tissue. Lamina densa consists of anchoring fibers made up of collagen Type VII, which binds to the collagen Type I and III of the extracellular matrix 49, 50. As the cells move from the basal layer to the surface, they show many biochemical and morphological changes. Morphologically, they become more flattened as they move from basal layer towards the surface.
Stratum spinosum consists of 10-20 layers of cells typically large in size, resembling spines. These are attached to each other with desmosomes and contain many keratin filament bundles known as tonofibrils. In the spinous layer, these cells show numerous contacts via desmosomes which are almost double in number as compared to the cells in the basal layer. There is a dramatic reduction in cell organelles as the cells move from the basal layer to the stratum granulosum. The nucleus of the cells becomes flattened. Excessive keratohyalin bodies and tonofibrils are seen in the cells. Numerous small electron-dense granules, also known as membrane coating granules or “Odland bodies”, are also observed in the cells of this layer. Odland bodies are small sub-cellular structures of size 200-300 nm. These are modified lysosomes, which contain ………………….Content available in the hard-copy of the website……………………………. Content available in the hard-copy of the website………..
Periobasics A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
In the stratum corneum, the cells become flattened and show signs of nucleus disintegration. Two terms, Orthokeratinization and para-keratinization are used to describe these changes. Para-keratinization is usually observed in the oral gingival epithelium which is characterized by an incomplete disintegration of the nucleus and cytoplasmic organelles. Ortho-keratinization is characterized by a complete disintegration of the nucleus and cytoplasmic organelles. It is found in the skin and may also be seen in the gingival epithelium. The degree of keratinization of stratum corneum reduces with age and with the onset of menopause 60.
The epithelium is firmly attached to the underlying connective tissue due to a high degree of integration. The basal cells show a large number of hemidesmosomes firmly attaching to the lamina densa of the basal lamina. This integration is further intensified by the presence of numerous serrated keratinocytes and cellular processes (pedicles) of these cells protruding into the connective tissue compartment. Along with acting as a physical barrier, the gingival epithelium also plays an important role in the innate immune response 61. Research has shown increased expression of integrins 62, intercellular adhesion molecule-1 (ICAM-1) 63, endothelial leukocytes adhesion molecule 1 (ELAM-1)64, 65 and vascular cell adhesion molecule (VCAM)-1 in the inflamed gingiva 62. Integrins are heterodimeric glycoproteins, which are involved in the attachment of cells to a large number of extracellular matrix ligands such as laminin, fibronectin, vitronectin, tenascin and osteopontin.
It has been demonstrated that expression of integrins especially those functioning as fibronectin receptors is increased in gingival epithelial cells during inflammation 66. The cell surface adhesion molecules belong to the immunoglobulin class. ICAM-1 molecule interacts with the leukocyte function associated with antigen-1 and is involved in the transmigration of neutrophil through the epithelium. ICAM-1 is expressed by keratinocytes of oral gingival and sulcular epithelium during gingival inflammation and its levels are elevated in periodontitis sites as compared to healthy sites 67. Expression of ELAM-1 by endothelial cells is increased under the influence of cytokines such as TNF-α, IL-1, and bacterial lipopolysaccharides. It plays an important role in the trans-endothelial migration of leukocytes during inflammation. Its expression has been shown to be increased in gingival inflammation 64, 65, 68. Toll-like receptors (TLRs) are important components of innate immunity. It has been demonstrated that pathogen-associated molecular patterns (PAMPs), shared by many different periodontopathogenic bacteria, stimulate the resident gingival epithelial cells to initiate inflammatory responses in a TLR-dependent manner 69. The expressions of TLRs have been reported in healthy as well as diseased periodontal tissues. Thus, these receptors actively participate in host-microbial interactions in periodontal diseases. A detailed description of TLRs and their role in host-microbial interactions has been discussed in “Host-microbial interactions in periodontal diseases”.
Melanocytes, Langerhan’s cells and Merkel cells in gingiva
Melanocytes:
Melanocytes are melanin pigment-producing cells. They have protective action against ultraviolet irradiation and have also been shown to be responsive to many immunological mediators 73. They have a protective role due to their ability to interact with active oxygen species (O 2-, H O , RO, ROO, etc.) 2 2, 74. Therefore, epithelial melanin pigmentation provides a defense barrier by acting as a binder for toxic products such as free radicals and polycyclic compounds 75. The distribution of oral melanin pigmentation is about 61% in the hard palate, 22% in the mucous membrane, and 15% in the tongue 76. The melanin pigmentation of the gingiva is normally observed in individuals of African, East-Asian or Hispanic ethnicity 77. Smoking also stimulates melanin production, leading to exceedingly evident intraoral pigmentation 78, 79. Excessive pigmentation of the gingiva is an esthetic problem and is treated by gingival depigmentation procedures.
Langerhan’s cells
Dendritic cells are potent antigen-presenting cells and may be the only cells capable of initiating the adaptive immune response. The dendritic cells in the epithelium are known as Langerhan’s cells. These serve as “sentinels” of the oral mucosa and inform the immune system not only about the entry of the pathogen, but also about the tolerance to self antigens and commensal microbes. These cells lack desmosomes and tonofilaments. They contain nuclei with clefts, lysosomes, centrioles, Golgi vesicles, a small amount of endoplasmic reticulum, and moderate numbers of mitochondria. A characteristic feature of these cells is the presence of g-specific granules or Birbeck granules (100 nm to 1 μm in size) first described by Birbeck et al. in 1961 80. Some of these granules may be seen associated with the cell membrane. These are rod-shaped and if the terminal vesicle is present, they assume the classic tennis-racket-like shape 81. The exact function of these granules is not clear, however, they have been associated with antigen trapping and presentation. On the basis of electron microscopic appearance, Langerhan’s cells have been divided into two types,
Type 1: They are pyramidal in shape and are highly dendritic with an electron-lucent cytoplasm. They have numerous Birbeck granules and are usually found in the suprabasal layers.
Type 2: These are spherical in shape and show fewer dendrites, a more electron-dense cytoplasm with fewer Birbeck granules. They are usually located in the basal layer.
Merkel cells
Merkel cells, Tactile cells, or Merkel-Ranvier cells are oval-shaped receptor cells found in the deeper layers of the epithelium. These cells have synaptic contacts with somatosensory afferents and are associated with the sense of light touch discrimination 82. These cells are associated with the development of Merkel cell carcinoma (MCC), which is a very aggressive small cell tumor of neuroendocrine origin, usually arising on sun-exposed parts of the skin.
Keratin expression in gingiva
As the cells move from the basal layer to the cornified layer, their morphological characters change. The keratin expression of gingival epithelium cells changes with their maturation. Keratins are fibrous proteins which take part in cornification of the stratified squamous epithelial tissue. There are 20 keratin polypeptides which have been divided into acidic and basic subfamilies. The gene coding for neutral-basic keratins are found on chromosome 12 (12q13.13), while the acidic keratins are found on chromosome 17 (17q21.2). The basic to neutral keratins have been numbered from K1 to K8 whereas the acidic keratins have been numbered from K9 to K19. Following expression of keratins is observed in stratified squamous epithelium,
- All basal cells in stratified epithelia express keratins K5 and K14.
- K1, K2, K10, and K11 are expressed in the suprabasal layers of keratinized stratified squamous epithelia 83.
- K4 and K13 expressions are observed in the suprabasal layers of non-keratinized and para-keratinized epithelia 84,85.
- K6 and K16 are expressed in highly proliferative epithelia.
- The oral gingival epithelium expresses K5, K14, K1, K2, K10, K11, K6, K8, K16, K18, and K19.
- The oral sulcular epithelium mostly expresses K5, K14, K4, K13, K6, K16, and K19.
- Junctional epithelium mostly expresses K5, K14, K13, and K19.
- Lining mucosa expresses K4/K13 pair which is associated with elasticity, whereas the masticatory mucosa expresses K1/K10 pair which is associated with rigidity.
Renewal of gingival epithelium:
The older cells are continuously replaced by new cells so that the integrity of the tissue can be maintained. In health, the rate of renewal of the epithelial cells equals to the rate of cell exfoliation, so that the total number of the cells remains constant. The renewal time or the turnover time is the time taken for complete renewal of the tissue. In other words, it is the time taken for the exfoliation of a number of cells corresponding to the total number of cells in the tissue, and the formation of the same number of cells through mitotic cell division.
There are a large number of biologically active substances that may stimulate or suppress ……………………Content available in the hard-copy of the website……………………………. Content available in the hard-copy of the website………..
Periobasics A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:
Sulcular epithelium
It is the epithelium which lines the gingival sulcus. Apically, it is bounded by the junctional epithelium and coronally it meets the outer gingival epithelium at the height of the free gingival margin. The sulcular epithelium is similar to the outer gingival. epithelium except for the lack of stratum corneum and it does not contain clearly defined stratum granulosum. Hence, this epithelium is non-keratinized. The lack of keratinization makes this area particularly susceptible to influences from microorganisms. Rete pegs are not present in the sulcular epithelium. The cells of the sulcular epithelium rarely show keratohyalin granules and membrane coating granules. The sulcular epithelium is relatively less permeable to water soluble substances as compared to junctional epithelium but is more permeable as compared to the oral epithelium. During gingival inflammation, the sulcular epithelium is densely infiltrated with PMN’s and lymphocytes. Due to infiltration by the immune cells, there is a loss of desmosomal attachment and widening of the intercellular spaces 96, 97.
Know More…
Oxygen consumption of gingiva:
In their classical studies Glickman et al. (1949, 1950) 98, 99 estimated the oxygen consumption by normal and healing gingiva using Warburg technique. They concluded that the oxygen quotient (QO ) of the normal gingiva is 1.6 ± 2 0.37. The consumption of the oxygen in the healing gingiva varies according to the nature of the microscopic tissue changes. They observed that during healing after gingivectomy, the maximum QO is achieved on the 2 fourteenth postoperative day. From the fourteenth day onwards till the twenty-first day, there is a decline in QO 2 to approximately the level of normal gingiva. The authors also discouraged the assumption that introduction of external oxygen could hasten the natural process of healing.
References
References are available in the hard-copy of the website.
Periobasics A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.

